Abstract
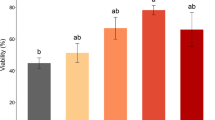
The aim of this study was to use more accurate techniques to investigate the effects of cryoprotectants (CPAs) and extenders on post-thaw sperm quality of Brycon orbignyanus and Prochilodus lineatus. Six freezing media comprising the combination of three CPAs (DMSO, methanol and methyl glycol) and two extenders (BTS and glucose) were used. Sperm was diluted in each medium, loaded into 0.5-mL straws, frozen in a nitrogen vapor vessel (dry-shipper), and stored in liquid nitrogen at −196 °C. Post-thaw sperm motility rate and velocities (curvilinear = VCL; straight line = VSL; average path = VAP) were evaluated using a computer-assisted sperm analyzer. Membrane integrity and mitochondrial function were determined using fluorochromes. Post-thaw quality was considered high when samples presented the following minimum values: 60 % motile sperm, 140 µm/s of VCL, 50 % intact sperm membrane and 50 % mitochondrial function integrity. High post-thaw quality was observed in B. orbignyanus sperm frozen in BTS–methyl glycol and in P. lineatus sperm frozen in BTS–methyl glycol, glucose–methyl glycol and glucose–methanol. All samples frozen in DMSO yielded low quality. The presence of ions in the BTS extender affected post-thaw sperm quality positively in B. orbignyanus and negatively in P. lineatus. Methyl glycol was the most suitable CPA for both fish species, leading to a good protection of cell membrane, mitochondrial function and motility apparatus during the cryopreservation process. For an improved protection, B. orbignyanus sperm should be frozen in an ionic freezing medium.
Similar content being viewed by others
References
Cabrita E, Alvarez R, Anel L, Rana KJ, Herraez MP (1998) Sublethal damage during cryopreservation of rainbow trout sperm. Cryobiology 37:245–253
Carneiro PCF, Azevedo HC, Santos JP, Maria AN (2012) Cryopreservation of tambaqui (Colossoma macropomum) semen: extenders, cryoprotectants, dilution ratios and freezing methods. Cryoletters 33(5):385–393
Cuevas-Uribe R, Leibo SP, Daly J, Tiersch TR (2011) Production of channel catfish with sperm cryopreserved by rapid non-equilibrium cooling. Cryobiology 63:186–197
Daly J, Tiersch TR (2012) Sources of variation in flow cytometric analysis of aquatic species sperm: the effect of cryoprotectants on flow cytometry scatter plots and subsequent population gating. Aquaculture 370–371:179–188
De Baulny BO, Labbé C, Maisse G (1999) Membrane integrity, mitochondrial activity, ATP content, and motility of the European catfish (Silurus glanis) testicular spermatozoa after freezing with different cryoprotectants. Cryobiology 39:177–184
Godinho HP, Viveiros ATM (2011) Current status of sperm cryopreservation of Brazilian Characiform fishes. In: Tiersch TR, Green CC (eds) Cryopreservation in aquatic species. World Aquaculture Society, Baton Rouge, pp 875–884
Gonçalves ACS, Nascimento AF, Costa AC, Leal MC, Viveiros ATM (2013) Initiation and suppression of sperm motility is osmolality-dependent in two South American fish species: streaked prochilod (Prochilodus lineatus) and piracanjuba (Brycon orbignyanus). Anim Reprod 10:62–70
Kopeika E, Kopeika J (2008) Variability of sperm quality after cryopreservation in fish. In: Alavi SMH, Cosson JJ, Coward K, Rafiee G (eds) Fish spermatology. Alpha Science Ltd, Oxford, pp 347–396
Li J, Liu Q, Zhang S (2006) Evaluation of the damage in fish spermatozoa cryopreservation. Chin J Oceanol Limnol 24:370–377
Lopez-Galvis DI (2014) Efeito da composição e osmolalidade do diluidor na qualidade do sêmen criopreservado de piracanjuba Brycon orbignyanus (Characiformes). Dissertation, Federal University of Lavras
Maria AN, Viveiros ATM, Freitas RTF, Oliveira AV (2006a) Extenders and cryoprotectants for cooling and freezing of piracanjuba (Brycon orbignyanus) semen, an endangered Brazilian teleost fish. Aquaculture 260:298–306
Maria AN, Viveiros ATM, Orfão LH, Oliveira AV, Moraes GF (2006b) Effects of cooling and freezing on sperm motility of the endangered fish piracanjuba Brycon orbignyanus (Characiformes, Characidae). Anim Reprod 3:55–60
Nascimento AF, Maria AN, Pessoa NO, Carvalho MAM, Viveiros ATM (2010) Out-of-season sperm cryopreserved in different media of the Amazonian freshwater fish pirapitinga (Piaractus brachypomus). Anim Reprod Sci 118:324–329
Nascimento AF, Gonçalves ACS, Reis Neto RV, Leal MC, Viveiros ATM (2012) Extender composition, osmolality, cryoprotectant and equilibration time effects on fresh sperm motility of two Characiformes fish: piracanjuba (Brycon orbignyanus) and streaked prochilod (Prochilodus lineatus). Anim Reprod 9(2):103–110
Orfão LH, Viveiros ATM, Maria AN, Silva FPC (2010) Criopreservação do sêmen de pacu Piaractus mesopotamicus: efeito de diluidores, crioprotetores e ativadores. In: Ciryno JEP, Furuya WM, Ribeito RP, Filho JDS (eds) Tópicos especiais em Biologia Aquática e Aqüicultura III. Sociedade Brasileira de Aqüicultura e Biologia Aquática, Jaboticabal, pp 161–167
Rosa RS, Lima FCT (2008) Os peixes ameaçados de extinção. In: Machado ABM, Drummond GM, Paglia AP (eds) Livro vermelho da fauna brasileira ameaçada de extinção. Ministério do Meio Ambiente/Fundação Biodiversitas, Brasília, pp 9–285
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, http://www.R-project.org, Accessed 30 Dec 2013
Van Zutphen LF, Baumans V, Beynen AC (2001) Principles of laboratory animal science, revised edn. Elsevier, Amsterdam, pp 1–428
Viveiros ATM, Maria AN, Orfão LH, Carvalho MAM, Nunes JF (2008) Powder coconut water (ACP-104) as extender for semen cryopreservation of Brazilian migratory fish species. Cybium 32 (8th ISRPF):215
Viveiros ATM, Orfão LH, Maria AN, Allaman IB (2009) A simple, inexpensive and successful freezing method for curimba Prochilodus lineatus (Characiformes) semen. Anim Reprod Sci 112:293–300
Viveiros ATM, Amaral TB, Orfão LH, Isaú ZA, Caneppele D, Leal MC (2011) Sperm cryopreservation of tiete tetra Brycon insignis (Characiformes): effects of cryoprotectants, extenders, thawing temperatures and activating agents on motility features. Aquac Res 42:858–865
Viveiros ATM, Gonçalves ACS, Di Chiacchio IM, Nascimento AF, Romagosa E Leal MC (2013) Gamete quality of streaked prochilod Prochilodus lineatus (Characiformes) after GnRHa and dopamine antagonist treatment. Zygote. doi:10.1017/S0967199413000440
Acknowledgments
This study received funding from the Brazilian fostering agencies CNPq (PQ 300994/2008-7; PQ 302434/2011-9; Grant 552471/2010-0), ANEEL P&D Furnas (017965) and FAPEMIG (Grant CVZ BPD 00216-11; PPM CVZ 00129-11), and from Czech programs CENAKVA CZ.1.05/2.1.00/01.0024, Strengthening of excellence scientific teams in USB FFPW CZ.1.07/2.3.00/20.0024, GACR P502/12/1973 and LO1205. This is part of A.F. Nascimento’s PhD project. The authors thank the undergraduate student M. Hamaue, MSc student T.R. Taffarel (UFLA), and the biologists D.M. Ribeiro and M.B. Goulart (Furnas) for assistance during the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viveiros, A.T.M., Nascimento, A.F., Leal, M.C. et al. Methyl glycol, methanol and DMSO effects on post-thaw motility, velocities, membrane integrity and mitochondrial function of Brycon orbignyanus and Prochilodus lineatus (Characiformes) sperm. Fish Physiol Biochem 41, 193–201 (2015). https://doi.org/10.1007/s10695-014-0016-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-0016-7