Abstract
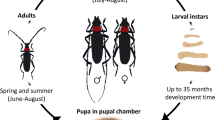
Kladothrips (Froggatt) is a genus of gall-inducing thrips that develop broods, and in some species, long-lived colonies within galls they form on phyllodes of Acacia in arid and semi-arid Australia. The gall interior is a stable environment for thrips in an otherwise inhospitable environment, but these conditions may also be favorable for fungal parasites. This fungal threat is corroborated by the observation that Kladothrips produce highly effective antifungal compounds. Here we investigated antifungal production in three Acacia thrips species, two gall-inducers: Kladothrips arotrum and Kladothrips tepperi, and one kleptoparasitic thrips, Koptothrips dyskritus. Using a spectrophotometer, the germination of a suspension of Cordyceps bassiana spores (an entomopathogenic fungus) can be detected by observing an abrupt increase in light absorption by the suspension. The addition of thrips exterior washes to these fungal spore suspensions resulted in significant delays in fungal germination for all three species. Foundresses in both Kladothrips species strongly delay fungal germination before their brood has matured. Young and maturing colonies with less than 50 adult individuals (the remainder of the brood are in juvenile stages of development) produced some antifungal effects but within the range produced by the foundress alone. Mature colonies (>100 adults) delayed germination for the duration of our observational window (48 h), suggesting a possible group size threshold for antifungal effectiveness. Koptothrips dyskritus antifungals were observed to be the strongest of the three species when <50 individuals were present. The strong antifungal abilities of the invading Ko. dyskritus would allow this species to invade older or damaged galls, which has been observed in the field. This pattern of antifungal activity in Acacia thrips suggests that effective defense against fungal pathogens is strongly associated with group size and colony maturity.


Similar content being viewed by others
References
Alteen H (2012) Antimicrobial producing streptomyces-like strains cultured from within social thrips colonies. Memorial University of Newfoundland and Labrador
Arnqvist G, Martensson T (1998) Measurement error in geometric morphometrics: empirical strategies to assess and reduce its impact on measures of shape. Acta Zool Acad Sci H 44(1–2):73–96
Baracchi D, Francese S, Turillazzi S (2011) Beyond the antipredatory defence: honey bee venom function as a component of social immunity. Toxicon 58(6–7):550–557
Beattie AJ, Turnbull CL, Hough T, Knox RB (1986) Antibiotic production: a possible function for the metapleural glands of ants (hymenoptera: Formicidae). Ann Entomol Soc Am 79(3):448–450
Blum MS (1991) Chemical ecology of the thysanoptera. Towards understanding Thysanoptera. USDA Forest Service General Technical Report NE-147:95-112
Blum MS, Foottit R, Fales HM (1992) Defensive chemistry and function of the anal exudate of the thrips Haplothrips leucanthemi. Comp Biochem Phys 102(1):209–211
Boos S, Meunier J, Pichon S, Kölliker M (2014) Maternal care provides antifungal protection to eggs in the European Earwig. Behav Ecol 25(4):754–761
Chapman TW, Crespi BJ, Kranz BD, Schwarz MP (2000) High relatedness and inbreeding at the origin of eusociality in gall-inducing thrips. Proc Natl Acad Sci USA 15 97(4):1648–1650
Chapman TW, Kranz BD, Bejah K, Morris DC, Schwarz MP, Crespi BJ (2002) The evolution of soldier reproduction in social thrips. Behav Ecol 13(4):519–525
Chapman TW, Francis-Geyer KL, Schwarz MP (2006) The impact of kleptoparasitic invasions on the evolution of gall-size in social and solitary Australian Acacia thrips. Insect Sci 13(5):391–400
Chapman TW, Crespi BJ, Perry SP (2008) The evolutionary ecology of eusociality in Australian gall thrips: a ‘model clades’ approach. In: Korb J, Heinze J (eds) Ecology of social evolution. Springer, Berlin, pp 57–83
Cremer S, Armitage SA, Schmid-Hempel P (2007) Social immunity. Curr Biol 7(16):R693–R702
Crespi BJ (1996) Comparative analysis of the origins and losses of eusociality: causal mosaics and historical uniqueness. In: Martins EP (ed) Phylogenies and the comparative method in animal behavior. Oxford University Press, Oxford, pp 253–287
Crespi BJ, Abbot P (1999) The behavioral ecology and evolution of kleptoparasitism in australian gall thrips. Fla Entomol 82(2):147–164
Crespi BJ, Carmean A, David A, Chapman TW (1997) Ecology and evolution of galling thrips and their allies. Annu Rev Entomol 42(1):51–71
Crespi BJ, Morris DC, Mound LA (2004) Evolution of ecological and behavioural diversity: Australian acacia thrips as model organisms, 1st edn. Australian Biological Resources Study and Australian National Insect Collection, CSIRO, Canberra, Australia
De Facci M, Wang H, Yuvaraj JK, Dublon IAN, Svensson GP, Chapman TW, Anderbrant O (2014) Chemical composition of anal droplets of the eusocial gall-inducing thrips Kladothrips intermedius. Chemoecology 24(3):85–94
Evans JD, Spivak M (2010) Socialized medicine: individual and communal disease barriers in honey bees. J Invertebr Pathol 103:S62–S72
Gillespie JP, Kanost MR, Trenczek T (1997) Biological mediators of insect immunity. Annu Rev Entomol 42(1):611–643
Gonsalves G (2010) Host exploitation and fidelity in acacia gall-invading parasites, Memorial University of Newfoundland and Labrador
Hamilton C, Lay F, Bulmer MS (2011) Subterranean termite prophylactic secretions and external antifungal defenses. J Insect Physiol 57(9):1259–1266
Hoggard SJ, Wilson PD, Beattie AJ, Stow AJ (2011) Social complexity and nesting habits are factors in the evolution of antimicrobial defences in wasps. PLoS ONE 6(7):e21763
Howard DF, Blum MS, Fales HM (1983) Defense in thrips: forbidding fruitiness of a lactone. Science 220(4594):335–336
Kaltenpoth M, Göttler W, Herzner G, Strohm E (2005) Symbiotic bacteria protect wasp larvae from fungal infestation. Curr Biol 15(5):475–479
Karatzoglou A, Feinerer I (2010) Kernel-based machine learning for fast text mining in R. Comput Stat Data Anal 54(2):290–297
Kranz BD, Schwarz MP, Wills TE, Chapman TW, Morris DC, Crespi BJ (2001) A fully reproductive fighting morph in a soldier clade of gall-inducing thrips (Oncothrips morrisi). Behav Ecol Sociobiol 50(2):151–161
Matsuura K, Matsunaga T (2015) Antifungal activity of a termite queen pheromone against egg-mimicking termite ball fungi. Ecol Res 30:93–100
McLeish MJ, Chapman TW (2007) The origin of soldiers in the gall-inducing thrips of Australia (Thysanoptera: phlaeothripidae). Aust J Entomol 46(4):300–304
McLeish MJ, Chapman TW, Crespi BJ (2006) Inbreeding ancestors: the role of sibmating in the social evolution of gall thrips. J Hered 97(1):31–38
Mcleish MJ, Chapman TW, Schwarz MP (2007) Host-driven diversification of gall-inducing acacia thrips and the aridification of Australia. BMC Biol 5:3
Morris DC, Mound LA, Schwarz MP, Crespi BJ (1999) Morphological phylogenetics of Australian gall-inducing thrips and their allies: the evolution of host-plant affiliations, domicile use and social behaviour. Syst Entomol 24(3):289–299
Morris DC, Schwarz MP, Cooper SJB, Mound LA (2002) Phylogenetics of Australian acacia thrips: the evolution of behaviour and ecology. Mol Phylogenet Evol 25(2):278–292
Mound LA (1971) Gall-forming thrips and allied species (Thysanoptera: phlaeothripinae) from Acacia trees in Australia. Bull Br Mus Nat 25:389–464
Mound LA, Heming BS, Palmer JM (1980) Phylogenetic relationships between the families of recent Thysanoptera Insecta. Zool J Linn Soc 69(2):111–141
Mound LA, Crespi B, Kranz B (1996) Gall-inducing Thysanoptera (Phlaeothripidae) on acacia phyllodes in Australia: host-plant relations and keys to genera and species. Invertebr Syst 10(6):1171–1198
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Method Ecol Evol 4(2):133–142
Nel P, Schmidt AR, Bässler C, Nel A (2013) Fossil thrips of the family Uzelothripidae suggest 53 million years of morphological and ecological stability. Acta Palaeontol Pol 58(3):609–614
Obin MS, Vander Meer RK (1985) Gaster flagging by fire ants (solenopsis spp.): functional significance of venom dispersal behavior. J Chem Ecol 11(12):1757–1768
R Development Core Team (2011) R: a language and environment for statistical computing. [computer program]. R Foundation for Statistical Computing, Vienna, Austria
Reber A, Purcell J, Buechel SD, Buri P, Chapuisat M (2011) Expression and impact of antifungal grooming in ants. J Evol Biol 24(5):954–964
Rosengaus RB, Savoie K (2002) The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc Natl Acad Sci USA 99(10):6838–6842
Rosengaus RB, Maxmen AB, Coates LE, Traniello JF (1998) Disease resistance: a benefit of sociality in the dampwood termite zootermopsis angusticollis (isoptera: Termopsidae). Behav Ecol Sociobiol 44(2):125–134
Rosengaus RB, Carlock DM, Traniello JFA, Lefebvre ML (2000) The social transmission of disease between adult male and female reproductives of the dampwood termite Zootermopsis angusticollis. Ethol Ecol Evol 12(4):419–433
Rosengaus RB, Traniello JFA, Lefebvre ML, Maxmen AB (2004) Fungistatic activity of the sternal gland secretion of the dampwood termite Zootermopsis angusticollis. Insectes Soc 51(3):259–264
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Shaw PA, Pell JK (2003) Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol 61:413–423
Smith SM, Beattie AJ, Gillings MR, Holley MP, Stow AJ, Turnbull CL, Wilson PD, Briscoe DA (2008) An enhanced miniaturized assay for antimicrobial prospecting. J Microbiol Methods 72(1):103–106
Stow A, Beattie A (2008) Chemical and genetic defenses against disease in insect societies. Brain Behav Immun 22(7):1009–1013
Stow A, Briscoe D, Gillings M, Holley M, Smith S, Leys R, Silberbauer T, Turnbull C, Beattie A (2007) Antimicrobial defences increase with sociality in bees. Biol Lett 3(4):422–424
Stow A, Turnbull C, Gillings M, Smith S, Holley M, Silberbauer L, Wilson PD, Briscoe D, Beattie A (2010) Differential antimicrobial activity in response to the entomopathogenic fungus Cordyceps in six Australian bee species. Aust J Entomol 49(2):145–149
Suzuki T, Haga K, Kodama S, Watanabe K, Kuwahara Y (1988) Secretion of thrips. II. Secretions of three gall-inhabiting thrips (Thysanoptera: Phlaeothripidae). Appl Entomol Zool 23(3):291–297
Suzuki T, Haga K, Tsutsumi T, Matsuyama S (2004) Analysis of anal secretions from Phlaeothripine thrips. J Chem Ecol 30(2):409–423
Tallamy DW (1984) Insect parental care. Bioscience 34(1):20–24
Teerling C, Pierce H Jr, Borden J, Gillespie D (1993) Identification and bioactivity of alarm pheromone in the western flower thrips Frankliniella occidentalis. J Chem Ecol 19(4):681–697
Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, Cremer S (2013) Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol 23(1):76–82
Tranter C, Graystock P, Shaw C, Lopes J, Hughes W (2014) Sanitizing the fortress: protection of ant brood and nest material by worker antibiotics. Behav Ecol Sociobiol 68(3):499–507
Turnbull C, Hoggard S, Gillings M, Palmer C, Stow A, Beattie D, Briscoe D, Smith S, Wilson P, Beattie A (2011) Antimicrobial strength increases with group size: implications for social evolution. Biol Lett 7(2):249–252
Turnbull C, Caravan H, Chapman T, Nipperess D, Dennison S, Schwarz M, Beattie A (2012a) Antifungal activity in thrips soldiers suggests a dual role for this caste. Biol Lett 8(4):526–529
Turnbull C, Wilson PD, Hoggard S, Gillings M, Palmer C, Smith S, Beattie D, Hussey S, Stow A, Beattie A (2012b) Primordial enemies: fungal pathogens in thrips societies. PLoS ONE 7(11):e49737
Uzel H (1905) Phoeothrips Tepperi nov.sp., ein Bewohner von Gallen auf Acacia aneura in Australia. Cas ces Spol Entomol 2:100–102
Vander Meer RK, Morel L (1995) Ant Queens deposit pheromones and antimicrobial agents on eggs. Naturwissenschaften 82:93–95
Veal DA, Trimble JE, Beattie AJ (1992) Antimicrobial properties of secretions from the metapleural glands of Myrmecia gulosa (the Australian bull ant). J Appl Bacteriol 72(3):188–194
Walker TN, Hughes WO (2009) Adaptive social immunity in leaf-cutting ants. Biol Lett 5(4):446–448
Wills T, Chapman T, Kranz B, Schwarz M (2001) Reproductive division of labour coevolves with gall size in Australian thrips with soldiers. Naturwissenschaften 88(12):526–529
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395
Acknowledgements
This work was funded by a Discovery Grant from the National Science and Engineering Research Council (NSERC) awarded to TWC, an Australian Research Council (ARC) Grant to AS and AB, a NSERC CGS-M, and Australian Endeavours Award awarded to PJC, and funding from Macquarie University’s IMQRES (cotutelle) and Memorial University SGS fellowship. We would like to thank Barbra Langille, Holly Caravan, and Laurence Mound for advice and aiding with field collections. Finally, we are grateful to Shannon Smith, Stephen Hoggard, Miranda Christopher, Paul Duckett, Liette Vandine, Sarah Collison, Jessica Thompson, and Siobhan Dennison for advice on lab and field techniques.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coates, P.J., Stow, A., Turnbull, C. et al. High density brood of Australian gall-inducing Acacia thrips aid in fungal control. Evol Ecol 31, 119–130 (2017). https://doi.org/10.1007/s10682-016-9874-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-016-9874-z