Abstract
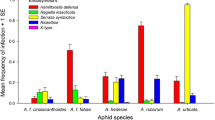
The host-associated differentiation (HAD) hypothesis states that higher trophic levels in parasitic associations should exhibit similar divergence in case of host sympatric speciation. We tested HAD on populations of Aphidius ervi the main parasitoid of the pea aphid Acyrthosiphon pisum, emerging from host populations specialized on either alfalfa or red clover. Host and parasitoid populations were assessed for genetic variation and structure, while considering geography, host plant and host aphid protective symbionts Regiella insecticola and Hamiltonella defensa as potential covariables. Cluster and hierarchical analyses were used to assess the contribution of these variables to population structure, based on genotyping pea aphids and associated A. ervi with microsatellites, and host aphid facultative symbionts with 16S rDNA markers. Pea aphid genotypes were clearly distributed in two groups closely corresponding with their plant origins, confirming strong plant associated differentiation of this aphid in North America. Overall parasitism by A. ervi averaged 21.5 % across samples, and many parasitized aphids producing a wasp hosted defensive bacteria, indicating partial or ineffective protective efficacy of these symbionts in the field. The A. ervi population genetic data failed to support differentiation according to the host plant association of their pea aphid host. Potential for parasitoid specialization was also explored in experiments where wasps from alfalfa and clover aphids were reciprocally transplanted on alternate hosts, the hypothesis being that wasp behaviour and parasitic stages should be most adapted to their host of origin. Results revealed higher probability of oviposition on the alfalfa aphids, but higher adult emergence success on red clover aphids, with no interaction as expected under HAD. We conclude that our study provides no support for the HAD in this system. We discuss factors that might impair A. ervi specialization on its divergent aphid hosts on alfalfa and clover.



Similar content being viewed by others
References
Abrahamson AG, Blair CP (2007) Sequential radiation through host race formation: herbivore diversity leads to diversity in natural enemies. In: Tilmon KJ (ed) Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects. University of California Press, Berkeley, pp 188–202
Aljanabi SM, Martinez I (1997) Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucl Acids Res 25:4692–4693
Allison PD (1999) Logistic regression using the SAS system—theory and application. SAS Institute Inc, Cary
Almohamad R, Verheggen FJ, Francis F et al (2008) Discrimination of parasitized aphids by a hoverfly predator: effects on larval performance, foraging, and oviposition behavior. Entomol Exp Appl 128:73–80
Angalet GW, Fuester R (1977) The Aphidius parasites of the pea aphid Acyrthosiphon pisum in the eastern half of the United States of America. Ann Entomol Soc Am 70:87–96
Barrette M, Wu GM, Brodeur J et al (2009) Testing competing measures of profitability for mobile resources. Oecologia 158:757–764
Battaglia D, Pennacchio F, Romano A et al (1995) The role of physical cues in the regulation of host recognition and acceptance behavior of Aphidius ervi Haliday (Hymenoptera, Braconidae). J Insect Behav 8:739–750
Baumann P, Baumann L, Lai CY et al (1995) Genetics, physiology and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Ann Rev Microbiol 49:55–94
Blackman RL, Eastop VF (1984) Aphids on the world’s crops. Interscience Publication, Toronto
Blair CP, Abrahamson WG, Jackman JA et al (2005) Cryptic speciation and host-race formation in a purportedly generalist tumbling flower beetle. Evolution 59:304–316
Bourguet D, Bethenod MT, Trouve C et al (2000) Host-plant diversity of the European corn borer (Ostrinia nubilalis): what value for sustainable transgenic insecticidal Bt maize? Proc R Soc B 267:1177–1184
Caillaud MC, Via S (2000) Specialized feeding behavior influences both ecological specialization and assortative mating in sympatric host races of pea aphids. Am Nat 156:606–621
Cameron PJ, Walker GP, Allan DJ (1981) Establishment of the introduced parasite Aphidius eadyi (Hymenoptera: Aphidiidae) in the north island of New Zealand, and its initial effect on pea aphid. N Z J Zool 8:105–112
Caro T (2007) Behavior and conservation: a bridge too far? Trends Ecol Evol 22:394–400
Charnov EL, Losdenhartogh RL, Jones WT et al (1981) Sex ratio evolution in a variable environment. Nature 289:27–33
Claridge MF, Denhollander J, Morgan JC (1985) The status of weed-associated populations of the brown planthopper, Nilaparvata lugens (Stål)—host race or biological species. Zool J Linn Soc 84:77–90
Cloutier C, Levesque CA, Eaves DM et al (1991) Maternal adjustment of sex-ratio in response to host size in the aphid parasitoid Ephedrus californicus. Can J Zool 69:1489–1495
Cloutier C, Duperron J, Tertuliano M et al (2000) Host instar, body size and fitness in the koinobiotic parasitoid Aphidius nigripes. Entomol Exp Appl 97:29–40
Colinet H, Salin C, Boivin G (2005) Host age and fitness-related traits in a koinobiont aphid parasitoid. Ecol Entomol 30:473–479
Cronin JT, Abrahamson AG (2001) Do parasitoids diversify in response to host-plant shifts by herbivorous insects? Ecol Entomol 26:347–355
Daza-Bustamante P, Fuentes-Contreras E, Rodriguez LC et al (2002) Behavioural differences between Aphidius ervi populations from two tritrophic systems are due to phenotypic plasticity. Entomol Exp Appl 104:321–328
Dion E, Zélé F, Simon JC et al (2011) Rapid evolution of parasitoids when faced with the symbiont-mediated resistance of their hosts. J Evol Biol 24:741–750
Dorchin N, Scott ER, Clarkin CE et al (2009) Behavioural, ecological and genetic evidence confirm the occurrence of host-associated differentiation in goldenrod gall-midges. J Evol Biol 22:729–739
Duchesne P, Turgeon J (2009) FLOCK: a method for quick mapping of admixture without source samples. Mol Ecol Resour 9:1333–1344
Ehrlich P, Raven P (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Emelianov I, Hernandes-Lopez A, Torrence M et al (2011) Fusion–fission experiments in Aphidius: evolutionary split without isolation in response to environmental bimodality. Heredity 106:798–807
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Farrell B (1988) “Inordinate fondness” explained: why are there so many beetles? Science 281:555–559
Feder JL, Forbes A (2010) Sequential speciation and the diversity of parasitic insects. Ecol Entomol 35:67–76
Feder JL, Chilcote CA, Bush GL (1988) Genetic differentiation between sympatric host races of the apple maggot fly Rhagoletis pomonella. Nature 336:61–64
Ferrari J, Godfray HCJ (2006) The maintenance of intraspecific biodiversity: the interplay of selection on resource use and on natural enemy resistance in the pea aphid. Ecol Res 21:9–16
Ferrari J, Darby AC, Daniell TJ et al (2004) Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol Entomol 29:60–65
Ferrari J, Godfray HCJ, Faulconbridge AS et al (2006) Population differentiation and genetic variation in host choice among pea aphids from eight host plant genera. Evolution 60:1574–1584
Forbes AA, Powell THQ, Stelinski LL et al (2009) Sequential sympatric speciation across trophic levels. Science 323:776–779
Frantz A, Plantegenest M, Mieuzet L et al (2006) Ecological specialization correlates with genotypic differentiation in sympatric host-populations of the pea aphid. J Evol Biol 19:392–401
Frantz A, Calcagno V, Mieuzet L et al (2009) Complex trait differentiation between host-populations of the pea aphid Acyrthosiphon pisum (Harris): implications for the evolution of ecological specialisation. Biol J Linn Soc 97:718–727
Fuller WA, Kim JK (2005) Hot deck imputation for the response model. Survey Methodology (Statistics Canada, Catalogue No. 12-001) 31:139–149
Gerling D, Roitberg BD, Mackauer M (1990) Instar-specific defense of the pea aphid, Acyrthosiphon pisum—influence on oviposition success of the parasite Aphelinus asychis (Hymenoptera, Aphelinidae). J Insect Behav 3:501–514
Guay JF, Boudreault S, Michaud D et al (2009) Impact of environmental stress on aphid clonal resistance to parasitoids: role of Hamiltonella defensa bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J Insect Physiol 55:919–926
Hales DF, Tomiuk J, Wohrmann K et al (1997) Evolutionary and genetic aspects of aphid biology: a review. Eur J Entomol 94:1–55
Hawthorne DJ, Via S (2001) Genetic linkage of ecological specialization and reproductive isolation in pea aphids. Nature 412:904–907
Henry LM, Gillespie DR, Roitberg BD (2005) Does mother really know best? Oviposition preference reduces reproductive performance in the generalist parasitoid Aphidius ervi. Entomol Exp Appl 116:167–174
Henry LM, Ma BO, Roitberg BD (2009) Size-mediated adaptive foraging: a host-selection strategy for insect parasitoids. Oecologia 161:433–445
Henter HJ (1995) The potential for coevolution in a host-parasitoid system. II. Genetic variation within a population of wasps in the ability to parasitize an aphid host. Evolution 49:439–445
Henter HJ, Via S (1995) The potential for coevolution in a host-parasitoid system. I. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution 49:427–438
Hufbauer RA (2001) Pea aphid-parasitoid interactions: have parasitoids adapted to differential resistance? Ecology 82:717–725
Hufbauer RA (2002) Evidence for nonadaptive evolution in parasitoid virulence following a biological control introduction. Ecol Appl 12:66–78
Hufbauer RA, Via S (1999) Evolution of an aphid-parasitoid interaction: variation in resistance to parasitism among aphid populations specialized on different plants. Evolution 53:1435–1445
Hufbauer RA, Bogdanowicz SM, Perez L et al (2001) Isolation and characterization of microsatellites in Aphidius ervi (Hymenoptera: Braconidae) and their applicability to related species. Mol Ecol Notes 1:197–199
Hufbauer RA, Bogdanowicz SM, Harrison RG (2004) The population genetics of a biological control introduction: mitochondrial DNA and microsatellite variation in native and introduced populations of Aphidius ervi, a parasitoid wasp. Mol Ecol 13:337–348
Janson E, Stireman JO, Singer MS et al (2008) Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 62:997–1012
Kunert G, Belz E, Simon JC et al (2010) Differences in defensive behaviour between host-adapted races of the pea aphid. Ecol Entomol 35:147–154
Langhof M, Meyhofer R, Poehling HM et al (2005) Measuring the field dispersal of Aphidius colemani (Hymenoptera: Braconidae). Agric Ecosyst Environ 107:137–143
Langley SA, Tilmon KJ, Cardinale BJ et al (2006) Learning by the parasitoid wasp, Aphidius ervi (Hymenoptera: Braconidae), alters individual fixed preferences for pea aphid color morphs. Oecologia 150:172–179
Le Ralec A, Anselme C, Outreman Y et al (2010) Evolutionary ecology of the interactions between aphids and their parasitoids. C R Biol 333:554–565
Leonardo TE, Muiru GT (2003) Facultative symbionts are associated with host plant specialization in pea aphid populations. Proc R Soc B 270:S209–S212
Libbrecht R, Gwynn DM, Fellowes MDE (2007) Aphidius ervi preferentially attacks the green morph of the pea aphid, Acyrthosiphon pisum. J Insect Behav 20:25–32
Losey JE, Ives AR, Harmon J et al (1997) A polymorphism maintained by opposite patterns of parasitism and predation. Nature 388:269–272
Lozier JD, Roderick GK, Mills NJ (2009) Molecular markers reveal strong geographic, but not host associated, genetic differentiation in Aphidius transcaspicus, a parasitoid of the aphid genus Hyalopterus. Bull Entomol Res 99:83–96
Mackauer M (1971) Acyrthosiphon pisum (Harris), pea aphid (Homoptera: Aphididae). In: Biological control programmes against insects and weeds in Canada 1959–1968. Technical communication no 4. Commonwealth Institute in Biological Control, Trinidad, pp 3–10
Mackauer M (1997) Growth and development in parasitoid wasps. In: Dettner K, Bauer G, Völkl W (eds) Vertical food web interactions: evolutionary patterns and driving forces. Ecological studies, vol 130. Springer, Berlin, pp 191–203
Mackauer M, Finlayson T (1967) The hymenopterous parasites (Hymenoptera: Aphidiidae) of the pea aphid in eastern North America. Can Entomol 99:1051–1082
Mackauer M, Michaud JP, Völkl W (1996) Host choice by aphidiid parasitoids (Hymenoptera: Aphidiidae): host recognition, host quality, and host value. Can Entomol 128:959–980
Medina RF (2005) The role of host-plant species in the differentiation of sympatric populations of hymenopteran parasitoids. Dissertation, University of Maryland, College Park
Michaud JP, Mackauer M (1994) The use of visual cues in host evaluation by aphidiid wasps. I. Comparison between three Aphidius parasitoids of the pea aphid. Entomol Exp Appl 70:273–283
Mitter C, Farrell B, Weigmann B (1988) The phylogenetic study of adaptive zones: has phytophagy promoted insect diversification? Am Nat 132:107–128
Moran NA, Dunbar HE (2006) Sexual acquisition of beneficial symbionts in aphids. Proc Natl Acad Sci USA 103:12803–12806
Morris RJ, Fellowes MDE (2002) Learning and natal host influence host preference, handling time and sex allocation behaviour in a pupal parasitoid. Behav Ecol Sociobiol 51:386–393
Morris DW, Kotler BP, Brown JS et al (2009) Behavioral indicators for conserving mammal diversity. Ann NY Acad Sci 1162:334–356
Nguyen TTA, Boudreault S, Michaud D et al (2008) Proteomes of the aphid Macrosiphum euphorbiae in its resistance and susceptibility responses to differently compatible parasitoids. Insect Biochem Mol Biol 38:730–739
Oliver KM, Russell JA, Moran NA et al (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA 100:1803–1807
Oliver KM, Moran NA, Hunter MS (2005) Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102:12795–12800
Oliver KM, Moran NA, Hunter MS (2006) Costs and benefits of a superinfection of facultative symbionts in aphids. Proc R Soc B 273:1273–1280
Oliver KM, Degnan PH, Burke GR et al (2010) Facultative symbionts of aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266
Peccoud J, Figueroa CC, Silva AX et al (2008) Host range expansion of an introduced insect pest through multiple colonizations of specialized clones. Mol Ecol 17:4608–4618
Peccoud J, Ollivier A, Plantegenest M et al (2009a) A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc Natl Acad Sci USA 106:7495–7500
Peccoud J, Simon JC, McLaughlin HJ et al (2009b) Post-Pleistocene radiation of the pea aphid complex revealed by rapidly evolving endosymbionts. Proc Natl Acad Sci USA 106:16315–16320
Powell W, Pennacchio F, Poppy G et al (1998) Strategies involved in the location of hosts by the parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae: Aphidiinea). Biol Control 11:104–112
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rice WR, Hostert EE (1993) Laboratory experiments on speciation—what have we learned in 40 years? Evolution 47:1637–1653
Roderick GK, Navajas M (2003) Genes in new environments: genetics and evolution in biological control. Nat Rev Genet 4:889–899
Russell JA, Moran NA (2005) Horizontal transfer of bacterial symbionts: heritability and fitness effects in a novel aphid host. Appl Environ Microbiol 71:7987–7994
Sandström J (1996) Temporal changes in host adaptation in the pea aphid, Acyrthosiphon pisum. Ecol Entomol 21:56–62
Sandström JP, Russell JA, White JP et al (2001) Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol Ecol 10:217–228
Schluter D (2000) The ecology of adaptative radiation. Oxford University Press, Oxford
Schwörer U, Völkl W (2001) Foraging behavior of Aphidius ervi (Haliday) (Hymenoptera: Braconidae: Aphidiinae) at different spatial scales: resource utilization and suboptimal weather conditions. Biol Control 21:111–119
Sequeira R, Mackauer M (1992) Nutritional ecology of an insect host-parasitoid association: the pea aphid—Aphidius ervi system. Ecology 73:183–189
Sequeira R, Mackauer M (1993) Seasonal variation in body size and offspring sex ratio in field populations of the parasitoid wasp, Aphidius ervi (Hymenoptera, Aphidiidae). Oikos 68:340–346
Simon JC, Carré S, Boutin M et al (2003) Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. Proc R Soc B 270:1703–1712
Simon JC, Sakurai M, Bonhomme J et al (2007) Elimination of a specialised facultative symbiont does not affect the reproductive mode of its aphid host. Ecol Entomol 32:296–301
Stireman JO, Nason JD, Heard SB et al (2006) Cascading host-associated genetic differentiation in parasitoids of phytophagous insects. Proc R Soc B 273:523–530
Sunnucks P, England PR, Taylor AC et al (1996) Microsatellite and chromosome evolution of parthenogenetic Sitobion aphids in Australia. Genetics 144:747–756
Takemoto H, Powell W, Pickett J et al (2009) Learning is involved in the response of parasitic wasps Aphidius ervi (Haliday) (Hymenoptera: Braconidae) to volatiles from a broad bean plant, Vicia faba (Fabaceae), infested by aphids Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Appl Entomol Zool 44:23–28
Tsuchida T, Koga R, Shibao H et al (2002) Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol Ecol 11:2123–2135
Tsuchida T, Koga R, Fukatsu T (2004) Host plant specialization governed by facultative symbiont. Science 303:1989
Via S (1991a) Specialized host plant performance of pea aphid clones is not altered by experience. Ecology 72:1420–1427
Via S (1991b) The genetic structure of host plant adaptation in a spatial patchwork - demographic variability among reciprocally transplanted pea aphid clones. Evolution 45:827–852
Via S (1999) Reproductive isolation between sympatric races of pea aphids. I. Gene flow restriction and habitat choice. Evolution 53:1446–1457
Via S (2001) Sympatric speciation in animals: the ugly duckling grows up. Trends Ecol Evol 16:381–390
Via S (2009) Natural selection in action during speciation. Proc Natl Acad Sci USA 106:9939–9946
Via S, Bouck AC, Skillman S (2000) Reproductive isolation between divergent races of pea aphids on two hosts. II. Selection against migrants and hybrids in the parental environments. Evolution 54:1626–1637
Vorburger C, Sandrock C, Gouskov A et al (2009) Genotypic variation and the role of defensive endosymbionts in an all-parthenogenetic host-parasitoid interaction. Evolution 63:1439–1450
Vorburger C, Gehrer L, Rodriguez P (2010) A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol Lett 6:109–111
Zilber-Rosenberg I, Rosenberg E (2008) Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev 32:723–735
Acknowledgments
This study was supported by a NSERC Discovery grant to Conrad Cloutier. We thank summer students (Sophie Laliberté, Joseph Moisan-De Serres, Anne Bogeto, Aurélie Guy and Claudia Zdenka-Ficher) for technical help with aphid and parasitoid collecting and rearing. We also thank Lucie Mieuzet for pea aphid genotyping and help with aphid symbiont detection, Jérôme Lemaître for help with data analysis, Pierre Duchesne from Université Laval for help with cluster analysis, Gaétan Daigle for help with AMOVA and Marie-Claude Gagnon for help with genetic analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bilodeau, E., Simon, JC., Guay, JF. et al. Does variation in host plant association and symbiont infection of pea aphid populations induce genetic and behaviour differentiation of its main parasitoid, Aphidius ervi?. Evol Ecol 27, 165–184 (2013). https://doi.org/10.1007/s10682-012-9577-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9577-z