Abstract
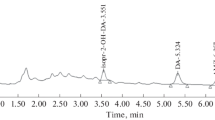
A new method for determination of hydrogen peroxide (H2O2) is described. H2O2 reacts with iodide in the presence of ammonium molybdate and vanillic acid resulting in the formation of iodovanillic acid which is quantified by reversed-phase high-performance liquid chromatography (HPLC) and detected by UV absorption at 280 nm. The method provides a detection limit of ∼0.1 μM and is easy to implement. Iodovanillic acid is stable, allowing multiple measurements of the same sample, and the separation power of the HPLC yields high selectivity against potential interferences. This method will be useful for many environmental applications. The method has been applied to quantify the photochemical production of H2O2 by aqueous suspensions of various minerals and soils.











Similar content being viewed by others
References
Andreozzi, R., Caprio, V., Insola, A., & Marotta, R. (1999). Advanced oxidation processes (AOP) for water purification and recovery. Catalysis Today, 53, 51–59.
Bader, H., Sturzenegger, V., & Hoigné, J. (1988). Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N, N-diethyl-p-phenylenediamine (DPD). Water Research, 22(9), 1109–1115.
Bou, R., Codony, R., Alba, T., Decker, E., & Guardiola, F. (2008). Determination of hydroperoxides in foods and biological samples by the ferrous oxidation–xylenol orange method: a review of factors that influence the methods performance. Analytical Biochemistry, 377, 1–15.
Boveris, A., Nozomo, O., & Chance, B. (1972). The cellular production of hydrogen peroxide. Biochemical Journal, 128, 617–630.
Boveris, A., Martino, E., & Stoppani, A. O. M. (1977). Evaluation of the horseradish peroxidase–scopoletin method for the measurement of hydrogen peroxide formation in biological system. Analytical Biochemistry, 80, 145–158.
Calvert, J. G., & Stockwell, W. R. (1983). Acid generation in the troposphere by gas-phase chemistry. Environmental Science and Technology, 17, 428A–443A.
Carraway, E. R., Hoffman, A. J., & Hoffmann, M. R. (1994). Photocatalytic oxidation of organic acids on quantum-sized semiconductor colloids. Environmental Science and Technology, 28, 786–793.
Chai, X.-S., Hou, Q. X., Lou, Q., & Zhu, J. Y. (2004). Rapid determination of hydrogen peroxide in wood pulp bleaching streams by a dual-wavelength spectroscopic method. Analytica Chimica Acta, 507, 281–284.
Copper, C., & Koubek, E. (1999). Kinetics of the molybdate and tungstate catalyzed oxidation of iodide by hydrogen peroxide. Inorganica Chimica Acta, 288, 229–232.
Corbett, J. T. (1989). The scopolitin assay for hydrogen peroxide a review and a better method. Journal of Biochemical and Biophysical Methods, 18, 297–308.
Gupta, B. L. (1973). Microdetermination techniques for H2O2 in irradiated solutions. Microchemical Journal, 18, 363–374.
Hartkamp, H., & Bachhausen, P. (1987). A method for the determination of hydrogen peroxide in air. Atmospheric Environment, 21, 2207–2213.
Hatchard, C. G., & Parker, C. A. (1956). A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical actinometer. Proceedings of the Royal Society of London. Series A: Mathematical and Physical Sciences, 235(1203), 518–536.
Hawk, J. P., McDaniel, E. L., Parish, T. D., & Simmons, K. E. (1972). Kinetic method for quantitative determination of individual peroxides in peroxide mixtures. Analytical Chemistry, 44(7), 1315–1317.
Hoffman, M. R., Martin, S. T., Choi, W., & Bhanemann, D. W. (1995). Environmental applications of semiconductor photocatalysis. Chemical Review, 95, 69–96.
Hurowitz, J. A., Tosca, N. J., McLennan, S. M., & Schoonen, M. A. A. (2007). Production of hydrogen peroxide in Martian and lunar soils. Earth and Planetary Science Letters, 225, 41–52.
Jen, J.-F., Leu, M.-F., & Yang, T. C. (1998). Determination of hydroxyl radicals in an advanced oxidation process with salicylic acid trapping and liquid chromatography. Journal of Chromatography. A, 796, 283–288.
Kelly, T. J., Daum, P. H., & Schwartz, S. E. (1985). Measurements of peroxides in cloud water and rain. Journal of Geophysical Research, 90, 7861–7871.
Kieber, R. J., & Helz, G. R. (1986). Two-method verification of hydrogen peroxide determinations in natural waters. Analytical Chemistry, 58, 2312–2315.
Klassen, N., Marchington, D., & McGowan, H. C. E. (1994). H2O2 determination by the I −3 method and by KMnO4 titration. Analytical Chemistry, 66, 2921–2925.
Klein, H. P., Horowitz, N. H., Levin, G. V., Oyama, V. I., Lederberg, J., Rich, A., Hubbard, J. S., Hobby, G. L., Straat, P. A., Berdahl, B. J., Carle, G. C., Brown, F. S., & Johnson, R. D. (1976). The viking biological investigation: preliminary results. Science, 194, 99–105.
Kleindienst, T. E., Shepson, P. B., Hodges, D. N., Nero, C. M., Arnts, R. R., Dasgupta, P. K., Hwang, H., Kok, G. L., Lind, J. A., Lazarus, A. L., Mackay, G. I., Mayne, L. K., & Schiff, H. I. (1988). Comparison of techniques for measurement of ambient levels of hydrogen peroxide. Environmental Science and Technology, 22, 53–61.
Korman, C., Bahnemann, D. W., & Hoffman, M. R. (1988). Photocatalytic production of H2O2 and organic peroxides in aqueous suspensions of TiO2, ZnO, and desert sand. Environmental Science and Technology, 22, 798–806.
Lee, J. H., Tang, I. N., Weinstein-Lloyd, B., & Halper, E. B. (1994). Improved nonenzymatic method for the determination of gas-phase peroxides. Environmental Science and Technology, 28, 1180–1185.
Li, W., Zhou, Q., & Hua, T. (2010). Removal of organic matter from landfill leachate by advanced oxidation processes: a review. International Journal of Chemical Engineering, Article ID 270532, 10 pages doi:10.1155/2010/270532.
Liu, J., Steinberg, S. M., & Johnson, B. J. (2003). A high performance liquid chromatography method for determination of gas-phase hydrogen peroxide in ambient air using Fenton’s chemistry. Chemosphere, 52, 815–823.
Madsen, B., & Kromis, M. S. (1984). Flow injection and photometric determination of hydrogen peroxide in rain-water with N-ethyl-N-(sulfopropyl)aniline sodium salt. Analytical Chemistry, 56, 2849–2850.
Mrowetz, M., & Selli, E. (2006). Photocatalytic degradation of formic and benzoic acids and hydrogen peroxide evolution in TiO2 and ZnO water suspensions. Journal of Photochemistry and Photobiology A: Chemistry, 180, 15–22.
Patrick, W., & Wagner, H. (1949). Determination of hydrogen peroxide in small concentrations. Analytical Chemistry, 21(10), 1279–1280.
Yen, A. S., Kim, S. S., Hecht, M. H., Fant, M. S., & Murry, B. (2000). Evidence that the reactivity of Martian soil is due to superoxide ions. Science, 289, 1909–1912.
Acknowledgments
The financial support of NASA EPSCoR (grant#NNX07AT65) is gratefully acknowledged. I thank Dr. Henry Sun of the Desert Research Institute for the gift of the Mojave Mars.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinberg, S.M. High-performance liquid chromatography method for determination of hydrogen peroxide in aqueous solution and application to simulated Martian soil and related materials. Environ Monit Assess 185, 3749–3757 (2013). https://doi.org/10.1007/s10661-012-2825-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2825-4