Abstract
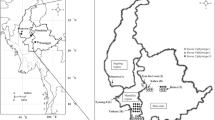
Moko disease caused by Ralstonia solanacearum (R. solanacearum) is a major disease affecting banana (Musa spp.) production. Although local reports suggested that this disease is widespread in Malaysia, molecular characterization of R. solanacearum strains associated with Moko disease in this country has not yet been done. During March 2011 to June 2012, 170 banana plants associated with Moko disease and the adjacent soil samples were collected in 12 different locations of five outbreak states in Peninsular Malaysia comprising Kedah, Selangor, Pahang, Negeri Sembilan and Johor, with disease incidence exceeding 80 % in some severely affected plantations. A total of 197 strains were identified as R. solanacearum-like colonies since they produced fluidal colonies that were white to pink coloration after incubation at 24 to 48 h at 29 °C on Kelman’s TZC agar medium, appeared as Gram-negative rods, and positive for potassium hydroxide (KOH), Kovacs oxidase, catalase and lipase activity on Tween 80 solution tests. Biovar tests disclosed that only 30 strains displayed characteristics of biovar 1 R. solanacearum associated with Moko disease, which was negative for utilization of disaccharides and hexose alcohols. Pathogenicity assay showed that these 30 strains were virulence towards Musa paradisiaca cv. Nipah explants with diverse degrees of virulence. Phylotype-specific multiplex PCR (Pmx-PCR) revealed all strains belonged to phylotype II displaying a 372 bp amplicon. Phylogenetic analyses of endoglucanase (egl) sequences clustered all 30 strains into phylotype II/4, together with the reference sequences strains from Peru (UW129, UW162 and UW163) and Colombia (UW070). Pooled rep-PCR fingerprinting method defined two major groups; cluster 1 (sub-group A and B) and cluster 2 (sub-group C), with 35 % average similarity coefficient within these two clusters. The sub-groups in cluster 1 were represented by strains from Kedah, Selangor, Negeri Sembilan and Johor; while cluster 2 sub-group was represented exclusively by strains of Pahang. To our knowledge, this is the first description of R. solanacearum phylotype II/4 in Malaysia and the Asian region. Our findings may expand constructive documentation and describe a better understanding on diversity of Malaysian R. solanacearum Moko-causing strains populations, thus will be useful for designing disease control strategies.






Similar content being viewed by others
References
Agrios, G. N. (2005). Plant pathology. San Diego: Elsevier Science.
Albuquerque, G. M., Santos, L. A., Félix, K. C., Rollemberg, C. L., Silva, A. M., Souza, E. B., Cellier, G., Prior, P., & Mariano, R. R. (2014). Moko disease-causing strains of Ralstonia solanacearum from Brazil extend known diversity in paraphyletic phylotype II. Phytopathology, 104(11), 1175–1182.
Aley, E. F. L. G. P., & Elphinstone, J. (1995). Culture media for Ralstonia solanacearum isolation, identification and maintenance. Fitopatologia, 30, 126–130.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410.
Álvarez, B., Biosca, E. G., & López, M. M. (2010). On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, 1, 267–279.
Arshiya, M., Suryawanshi, A., More, D., & Baig, M. M. V. (2014). Repetitive PCR based detection of Genetic Diversity in Xanthomonas axonopodis pv citri Strains. Journal of Applied Biology and Biotechnology, 2(01), 017–022.
Castillo, J. A., & Greenberg, J. T. (2007). Evolutionary dynamics of Ralstonia solanacearum. Applied and Environmental Microbiology, 73, 1225–1238.
Cellier, G., Remenant, B., Chiroleu, F., Lefeuvre, P., & Prior, P. (2012). Phylogeny and population structure of brown rot-and Moko disease-causing strains of Ralstonia solanacearum phylotype II. Applied and Environmental Microbiology, 78(7), 2367–2375.
de Bruijn, F. J. (1992). Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Applied and Environmental Microbiology, 58, 2180–2187.
Denny, T. (2006). Plant pathogenic Ralstonia species. In Plant-associated bacteria (pp. 573–644). Netherlands: Springer.
Drummond, A. J., & Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7(1), 214.
Engering, A., Hogerwerf, L., & Slingenbergh, J. (2013). Pathogen–host–environment interplay and disease emergence. Emerging Microbes and Infections, 2, e5.
Eyres, N., Hammond, N., & Mackie, A. (2001). Moko disease Ralstonia solanacearum (Race 2, Biovar 1). In Department of Agriculture and Food Factsheet, Government of Western Australia.
Fegan, M. (2006). Bacterial wilt of banana–diagnostics manual draft copy. In Plant Health Australia and the CRC for tropical plant protection. St Lucia, Australia.
Fegan, M., & Prior, P. (2005). How complex is the ‘Ralstonia solanacearum’ species complex? In: Bacterial wilt: the disease and the Ralstonia solanacearum species complex (pp. 49–61). American Phytopathological Society.
Fegan, M., & Prior, P. (2006). Diverse members of the Ralstonia solanacearum species complex cause bacterial wilts of banana. Australasian Plant Pathology, 35(2), 93–101.
Fonseca, N. R., Guimarães, L. M. S., Hermenegildo, P. S., Teixeira, R. U., Lopes, C. A., & Alfenas, A. C. (2013). Molecular characterization of Ralstonia solanacearum infecting Eucalyptus spp. in Brazil. Forest Pathology, 44(2), 107–116.
French, E. R., & Sequeira, L. (1969). Strains of Pseudomonas solanacearum from Central and South America: A comparative study. Phytopathology, 60, 506–512.
Gouy, M., Guindon, S., & Gascuel, O. (2010). SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution, 27(2), 221–224.
Hayward, A. C., & Hartmann, G. L. (1994). Pseudomonas solanacearum. Pathogenesis and host specificity in plant diseases: histopathological, biochemical, genetic and molecular bases, 1, 139–151.
Hayward, A. C. (1964). Characteristics of Pseudomonas solanacearum. Journal of Applied Bacteriology, 27, 265–277.
Horita, M., & Tsuchiya, K. (2001). Genetic diversity of Japanese strains of Ralstonia solanacearum. Phytopathology, 91, 399–407.
Horita, M., Suga, Y., Ooshiro, A., & Tsuchiya, K. (2010). Analysis of genetic and biological characters of Japanese potato strains of Ralstonia solanacearum. Journal of General Plant Pathology, 76(3), 196–207.
Ji, P., Allen, C., Sanchez-Perez, A., Yao, J., Elphinstone, J. G., Jones, J. B., & Momol, M. T. (2007). New diversity of Ralstonia solanacearum strains associated with vegetable and ornamental crops in Florida. Plant Disease, 91(2), 195–203.
Katoh, K., Misawa, K., Kuma, K. I., & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30(14), 3059–3066.
Kelman, A. (1954). The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance in a tetrazolium medium. Phytopathology, 44, 693–695.
Khakvar, R., Kamaruzaman, S., Wong, M. Y., Radu, S., Jones, J., & Thong, K. L. (2008). Genomic diversity of Ralstonia solanacearum strains isolated from banana farms in West Malaysia. Plant Pathology Journal, 7, 162–167.
Khakvar, R., Sijam, K., Jones, J., & Thong, K. L. (2011). Comparative diversity analysis of Ralstonia solanacearum strains in solanaceae farms. In Proceedings of International Conference on Life Science and Technology, (3, pp 38–42).
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J., & Higgins, D. G. (2007). ClustalW and ClustalX version 2. Bioinformatics, 23(21), 2947–2948.
Lemessa, F., Zeller, W., & Negeri, D. (2010). Genetic diversity among strains of Ralstonia solanacearum from Ethiopia assessed by repetitive sequence-based polymerase chain reaction (rep-PCR). EJAST, 1, 17–26.
Louws, F. J., Fulbright, D. W., Stephens, C. T., & De Bruijn, F. J. (1994). Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Applied and Environmental Microbiology, 60, 2286–2295.
Louws, F. J., Rademaker, J. L. W., & De Bruijn, F. J. (1999). The three Ds of PCR-based genomic analysis of phytobacteria: diversity, detection, and disease diagnosis. Annual Review of Phytopathology, 37, 81–125.
Mokhtarud-din, H., & William, R. (2011). Status of banana cultivation and disease incidences in Malaysia. In Abstract of the Workshop on Integrated Approaches in Banana Disease Management, 22th March, (pp. 5). Serdang.
bin Nik Hassan, N. M. M. (2003). Banana R&D in Malaysia: Updates and highlights. In Proceedings of the 21st BAPNET Steering Committee Meeting, (12, pp 75–79). Jakarta.
Norman, D. J., Zapata, M., Gabriel, D. W., Duan, Y. P., Yuen, J. M., Mangravita-Novo, A., & Donahoo, R. S. (2009). Genetic diversity and host range variation of Ralstonia solanacearum strains entering North America. Phytopathology, 99, 1070–1077.
Nouri, S., Bahar, M., & Fegan, M. (2009). Diversity of Ralstonia solanacearum causing potato bacterial wilt in Iran and the first record of phylotype II/biovar 2T strains outside South America. Plant Pathology, 58, 243–249.
Pasanen, T., Koskela, S., Mero, S., Tarkka, E., Tissari, P., Vaara, M., & Kirveskari, J. (2014). Rapid molecular characterization of Acinetobacter baumannii clones with rep-PCR and evaluation of carbapenemase genes by new multiplex PCR in Hospital District of Helsinki and Uusimaa. PloS One, 9(1), e85854.
Poussier, S., Prior, P., Luisetti, J., Hayward, C., & Fegan, M. (2000). Partial sequencing of the hrpB and endoglucanase genes confirms and expands the known diversity within the Ralstonia solanacearum species complex. Systematic and Applied Microbiology, 23, 479–486.
Rodrigues, L. M., Destéfano, S. A., Diniz, M. C. T., Comparoni, R., & Rodrigues Neto, J. (2011). Pathogenicity of Brazilian strains of Ralstonia solanacearum in Strelitzia reginae seedlings. Tropical Plant Pathology, 36, 409–413.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12), 1572–1574.
Sahilah, A. M., Tosiah, S., Radu, S., Ooi, W. L., Queen, C. Y. K., Jeffrey, L. S. H., Chua, S. C., & Senawi, M. T. (2005). Typing of Ralstonia solanacearum isolated from tomato by antibiotic susceptibility, plasmid profiling and PCR-based techniques of RAPD and ERIC. Journal of Tropical Agriculture and Food Science, 33, 73–82.
Santana, B. G., Lopes, C. A., Alvarez, E., Barreto, C. C., Allen, C., & Quirino, B. F. (2012). Diversity of Brazilian biovar 2 strains of Ralstonia solanacearum. Journal of General Plant Pathology, 78(3), 190–200.
Schaad N. W., Jones J. B., & Chun, W. (2001). In Schaad NW, Jones JB, & Chun W (eds.), Laboratory guide for identification of plant pathogenic bacteria. St. Paul, Minnesota: American Phytopathological Society Press.
Tengku Ab. Malik, T. M., Rozeita, L., Maimun, T. & Umi Kalsum, B. (2011). Status of banana diseases research in Malaysia. In Abstract of the Workshop on Integrated Approaches in Banana Disease Management, 22th March, (pp. 5). Serdang.
Thwaites, R., Mansfield, J., Eden-Green, S., & Seal, S. (1999). RAPD and rep PCR-based fingerprinting of vascular bacterial pathogens of Musa spp. Plant Pathology, 48, 121–128.
Villa, J. E., Tsuchiya, K., Horita, M., Opina, N., & Hyakumachi, M. (2005). Phylogenetic relationships of Ralstonia solanacearum species complex strains from Asia and other continents based on 16S rDNA, endoglucanase, and hrpB gene sequences. Journal of General Plant Pathology, 71, 39–46.
Wicker, E., Grassart, L., Coranson-Beaudu, R., Mian, D., Guilbaud, C., Fegan, M., & Prior, P. (2007). Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Applied and Environmental Microbiology, 73(21), 6790–6801.
Wicker, E., Grassart, L., Coranson-Beaudua, R., Mianc, D., & Prior, P. (2009). Epidemiological evidence for the emergence of a new pathogenic variant of Ralstonia solanacearum in Martinique (French West Indies). Plant Pathology, 58, 853–861.
Wicker, E., Lefeuvre, P., de Cambiaire, J. C., Lemaire, C., Poussier, S., & Prior, P. (2011). Contrasting recombination patterns and demographic histories of the plant pathogen Ralstonia solanacearum inferred from MLSA. The ISME Journal, 6(5), 961–974.
Winstead, N. N., & Kelman, A. (1952). Inoculation techniques for evaluating resistance to Pseudomonas solanacearum. Phytopathology, 42, 628–634.
Zulperi, D., & Sijam, K. (2014). First report of Ralstonia solanacearum race 2 biovar 1 causing Moko disease of banana in Malaysia. Plant Disease, 98, 275.
Zulperi, D., Sijam, K., Ahmad, M., Zainal, A., Awang, Y., & Sulaiman Rashid, T. (2014a). Occurrence of Ralstonia solanacearum race 2 biovar 1 associated with Moko disease of banana (Musa paradisiaca cv. Nipah) in Malaysia. Journal of Phytopathology, 162(10), 697–702.
Zulperi, D., Sijam, K., Mior Ahmad, Z. A., Awang, Y., & Mohd Hata, E. (2014b). Phylotype classification of Ralstonia solanacearum biovar 1 strains isolated from banana (Musa spp.) in Malaysia. Archives of Phytopathology and Plant Protection, 47(19), 2352–2364.
Acknowledgments
This work was supported by a grant from UPM (9316300). We thank Professor Mark Gleason from Iowa State University for his critical review of this manuscript, insights and suggestions. We are really grateful to the staffs from Bacteriology Laboratory, Department of Plant Protection, UPM and Department of Agriculture, Malaysia, for assistance with sampling and isolation procedures.
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zulperi, D., Sijam, K., Ahmad, Z.A.M. et al. Genetic diversity of Ralstonia solanacearum phylotype II sequevar 4 strains associated with Moko disease of banana (Musa spp.) in Peninsular Malaysia. Eur J Plant Pathol 144, 257–270 (2016). https://doi.org/10.1007/s10658-015-0764-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0764-y