Summary
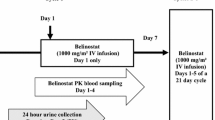
Background Belinostat, a potent pan-inhibitor of histone deacetylase (HDAC) enzymes, is approved in the United States (US) for relapsed/refractory peripheral T-cell lymphoma. In nonclinical studies, bile and feces were identified as the predominant elimination routes (50–70 %), with renal excretion accounting for ~30–50 %. A Phase 1 human mass balance study was conducted to identify species-dependent variations in belinostat metabolism and elimination. Methods Patients received a single 30-min intravenous (IV) infusion of 14C-labeled belinostat (1500 mg). Venous blood samples and pooled urine and fecal samples were evaluated using liquid chromatography - tandem mass spectroscopy for belinostat and metabolite concentrations pre-infusion through 7 days post-infusion. Total radioactivity was determined using liquid scintillation counting. Continued treatment with nonradiolabled belinostat (1000 mg/m2 on Days 1–5 every 21 days) was permitted. Results Belinostat was extensively metabolized and mostly cleared from plasma within 8 h (N = 6), indicating that metabolism is the primary route of elimination. Systemic exposure for the 5 major metabolites was >20 % of parent, with belinostat glucuronide the predominant metabolite. Mean recovery of radioactive belinostat was 94.5 % ± 4.0 %, with the majority excreted within 48 and 96 h in urine and feces, respectively. Renal elimination was the principal excretion route (mean 84.8 % ± 9.8 % of total dose); fecal excretion accounted for 9.7 % ± 6.5 %. Belinostat was well tolerated, with mostly mild to moderate adverse events and no treatment-related severe/serious events. Conclusion Mass balance was achieved (~95 % mean recovery), with metabolism identified as the primary route of elimination. Radioactivity was predominantly excreted renally as belinostat metabolites.



Similar content being viewed by others
References
Qian X, LaRochelle WJ, Ara G, et al. (2006) Activity of PXD101, a histone deacetylase inhibitor, in preclinical ovarian cancer studies. Mol Cancer Ther 5(8):2086–2095
Plumb JA, Finn PW, Williams RJ, et al. (2003) Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel deacetylase inhibitor PXD101. Mol Cancer Ther 2(8):721–728
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5(9):769–784
O’Connor OA, Horwitz S, Masszi T, et al. (2015) Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol 33:2492–2499
Steele NL, Plumb JA, Vidal L, et al. (2008) A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res 14(3):804–810
Beumer JH, Beijnen JH, Schellens JH (2006) Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinetics 45(1):33–58
Penner N, Klunk LJ, Prakash C (2009) Human radiolabeled mass balance studies: objectives, utilities and limitations. Biopharm Drug Dispos 30(4):185–203
Wang LZ, Ramirez J, Yeo W, et al. (2013) Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients. PLoS One 8:1–10
Kiesel BF, Parise RA, Tjornelund J, et al. (2013) LC-MS/MS assay for the quantitation of the HDAC inhibitor belinostat and five major metabolites in human plasma. J Pharm Biomed Anal 81-82:89–98
Bosma PJ, Chowdhury JR, Bakker C, et al. (1995) The genetic basis of reduced expression of bilirubin UDP-glucuronosyltransferase in gilbert’s syndrome. N Engl J Med 333:1171–1175
Goey AK, Sissung TM, Peer CJ et al (2015) Effects of UGT1A1 genotype on the pharmacokinetics, pharmacodynamics and toxicities of belinostat administered by 48 h continuous infusion in patients with cancer. J Clin Pharmacol (ahead of print).
Acknowledgments
Medical writing assistance was provided by Lindsey Lozano.
Research funding for this study was provided by Spectrum Pharmaceuticals.
Previously presented in part at the 2015 annual conference of the American Association of Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Emiliano Calvo, MD, PhD, Valentina Boni, MD, PhD, and Lina García-Cañamaque, MD declare that they have no conflict of interest. Guru Reddy, PhD, Tao Song, PhD, Mi Rim Choi, MD, and Lee F. Allen, MD, PhD are employees and stockholders of Spectrum Pharmaceuticals, Inc., and Jette Tjornelund, PhD was an employee and stockholder of Onxeo at the time of study conduct.
Rights and permissions
About this article
Cite this article
Calvo, E., Reddy, G., Boni, V. et al. Pharmacokinetics, metabolism, and excretion of 14C-labeled belinostat in patients with recurrent or progressive malignancies. Invest New Drugs 34, 193–201 (2016). https://doi.org/10.1007/s10637-015-0321-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0321-8