Summary
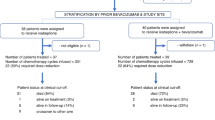
Background/Purpose This study was designed to evaluate the response and toxicity of sorafenib alone or when combined with carboplatin and paclitaxel in patients with platinum-sensitive, recurrent ovarian cancer, fallopian tube cancer, or primary peritoneal cancer (EOC). Methods Patients with recurrent platinum-sensitive EOC with no more than 2 prior courses of chemotherapy were randomized to single-agent sorafenib 400 mg twice daily or combination sorafenib 400 mg bid (days 2–19) with IV carboplatin (AUC 6) and IV paclitaxel 175 mg/m2 (S+C/T) every 3 weeks. Single agent sorafenib could cross over to combination upon progression. Results Patients were initially randomized to either arm, however, due to poor accrual, sorafenib arm was prematurely closed. A total of 13 patients were evaluable for response to sorafenib and 23 patients were evaluable for response to S+C/T. Objective response rate (RR) was 15 % for patients on sorafenib vs. 61 % for patients on S+C/T (p = 0.014); stable disease was seen in 62 % and 35 %, respectively. Clinical benefit rate (CBR) at 4 months (mos.) was 69 % for S and 65 % for S+C/T. The median progression free survival was 5.6 months on sorafenib vs. 16.8 months on S+C/T (p = 0.012) and there was no significant difference of overall survival between two arms (p = 0.974) with median overall survival 25.6 months under sorafenib vs. 25.9 months on S+C/T. Patients remained on trial for a median of 7.8 cycles on sorafenib and 5.4 cycles on S+C/T. Conclusion Sorafenib, alone or in combination with carboplatin and paclitaxel, has activity in patients with platinum-sensitive EOC. Sorafenib in combination with carboplatin and paclitaxel improved RR and PFS; however, there were increased grade and frequencies of toxicities.



Similar content being viewed by others
References
Van Cutsem E et al (2009) Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol Off J Eur Soc Med Oncol 20:1842–1847. doi:10.1093/annonc/mdp233
Aghajanian C et al (2012) OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol Off J Am Soc Clin Oncol 30:2039–2045. doi:10.1200/jco.2012.42.0505
Wilhelm S et al (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5:835–844. doi:10.1038/nrd2130
Escudier B et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134. doi:10.1056/NEJMoa060655
Llovet JM et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390. doi:10.1056/NEJMoa0708857
Chien CH, Chow SN (1993) Point mutation of the ras oncogene in human ovarian cancer. DNA Cell Biol 12:623–627
McPhillips F et al (2001) Association of c-Raf expression with survival and its targeting with antisense oligonucleotides in ovarian cancer. Br J Cancer 85:1753–1758. doi:10.1054/bjoc.2001.2139
Davies H et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954. doi:10.1038/nature00766
Lee JM et al (2010) Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer 102:495–499. doi:10.1038/sj.bjc.6605514
Welch SA et al (2010) Sorafenib in combination with gemcitabine in recurrent epithelial ovarian cancer: a study of the Princess Margaret Hospital Phase II Consortium. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc 20:787–793. doi:10.1111/IGC.0b013e3181e273a8
Ramasubbaiah R et al (2011) Sorafenib in combination with weekly topotecan in recurrent ovarian cancer, a phase I/II study of the Hoosier Oncology Group. Gynecol Oncol 123:499–504. doi:10.1016/j.ygyno.2011.08.033
Matei D et al (2011) Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol Off J Am Soc Clin Oncol 29:69–75. doi:10.1200/JCO.2009.26.7856
Therasse P et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Brown LD et al (2001) Interval estimation for a binomial proportion - Comment - Rejoinder. Stat Sci 16:101–133
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Marquez CB et al (2009) Multiple keratoacanthomas arising in the setting of sorafenib therapy: novel chemoprophylaxis with bexarotene. Cancer Control 16:66–69
Rose PG, Fusco N, Fluellen L, Rodriguez M (1998) Second-line therapy with paclitaxel and carboplatin for recurrent disease following first-line therapy with paclitaxel and platinum in ovarian or peritoneal carcinoma. J Clin Oncol Off J Am Soc Clin Oncol 16:1494–1497
Wheler JJ et al (2012) Risk of serious toxicity in 1181 patients treated in phase I clinical trials of predominantly targeted anticancer drugs: the M. D. Anderson Cancer Center experience. Ann Oncol Off J Eur Soc Med Oncol 23:1963–1967. doi:10.1093/annonc/mds027
Arnault JP et al (2009) Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol Off J Am Soc Clin Oncol 27:e59–e61. doi:10.1200/JCO.2009.23.4823
Arnault JP et al (2012) Skin tumors induced by sorafenib; paradoxic RAS-RAF pathway activation and oncogenic mutations of HRAS, TP53, and TGFBR1. Clin Cancer Res Off J Am Assoc Cancer Res 18:263–272. doi:10.1158/1078-0432.CCR-11-1344
Dubauskas Z et al (2009) Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer 7:20–23. doi:10.3816/CGC.2009.n.003
Hong DS et al (2008) Multiple squamous cell carcinomas of the skin after therapy with sorafenib combined with tipifarnib. Arch Dermatol 144:779–782. doi:10.1001/archderm.144.6.779
Bodnar L, Górnas M, Szczylik C (2011) Sorafenib as a third line therapy in patients with epithelial ovarian cancer or primary peritoneal cancer: a phase II study. Gynecol Oncol 123:33–36. doi:10.1016/j.ygyno.2011.06.019
Burger RA et al (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–2483. doi:10.1056/NEJMoa1104390
Perren TJ et al (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496. doi:10.1056/NEJMoa1103799
Stark D et al (2013) Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality-of-life outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) phase 3 randomised trial. Lancet Oncol 14:236–243. doi:10.1016/S1470-2045(12)70567-3
Acknowledgments
Supported by grant U01-CA62502 (PI: A. Dowlati) from the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwandt, A., von Gruenigen, V.E., Wenham, R.M. et al. Randomized phase II trial of sorafenib alone or in combination with carboplatin/paclitaxel in women with recurrent platinum sensitive epithelial ovarian, peritoneal, or fallopian tube cancer. Invest New Drugs 32, 729–738 (2014). https://doi.org/10.1007/s10637-014-0078-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0078-5