Summary
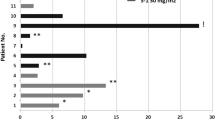
Background This phase Ib study was designed to determine the maximum tolerated doses (MTD) and dose limiting toxicities (DLTs) of irinotecan and cetuximab with sorafenib. Secondary objectives included characterizing the pharmacokinetics and pharmacodynamics and evaluating preliminary antitumor activity in patients with advanced colorectal cancer (CRC). Methods Patients with metastatic, pretreated CRC were treated at five dose levels. Results Eighteen patients were recruited with median age 56.5 years. In the first five patients treated, 2 irinotecan related DLTs were observed. With reduced dose intensity irinotecan, there were no further DLTs. The most common toxicities were diarrhea, nausea/vomiting, fatigue, anorexia and rash. DLTs included neutropenia and thrombocytopenia. Two patients had partial responses (one with a KRAS mutation) and 8 had stable disease (8–36 weeks). The median progression free survival (PFS) and overall survival (OS) were 2.5 and 4.7 months respectively. Pharmacokinetic analyses suggest sorafenib and metabolite exposure correlate with OS and DLTs. Conclusions The recommended phase II dose (RP2D) is irinotecan 100 mg/m2 i.v. days 1, 8; cetuximab 400 mg/m2 i.v. days 1 and 250 mg/m2 i.v. weekly; and sorafenib 400 mg orally twice daily in advanced, pretreated CRC. The combination resulted in a modest response rate.


Similar content being viewed by others
References
Fang JY, Richardson BC (2005) The MAPK signalling pathways and colorectal cancer. Lancet Oncol 6(5):322–327
Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM (1995) Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 55(18):3964–3968
Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G (2006) Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 281(2):951–961
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357(20):2040–2048
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26(14):2311–2319
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360(14):1408–1417
Brink M, de Goeij AF, Weijenberg MP, Roemen GM, Lentjes MH, Pachen MM, Smits KM, de Bruine AP, Goldbohm RA, van den Brandt PA (2003) K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis 24(4):703–710
Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML (2000) Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev 9(11):1193–1197
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25(13):1658–1664
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765
Dasari A, Messersmith WA (2010) New strategies in colorectal cancer: biomarkers of response to epidermal growth factor receptor monoclonal antibodies and potential therapeutic targets in phosphoinositide 3-kinase and mitogen-activated protein kinase pathways. Clin Cancer Res 16(15):3811–3818
Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, Deeter R et al (2009) A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 27(5):672–680
Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA, Richel DJ et al (2009) Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 360(6):563–572
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y et al (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109
Wilhelm S, Housley T, Rong H et al. (2003) The novel Raf inhibitor BAY 43–9006 blocks signaling and proliferation in BRAF mutant and wildtype melanoma and colorectal tumor cell lines. Proc Am Assoc Cancer Res;44:164 (Abstract 106609).
Martinelli E, Troiani T, Morgillo F, Rodolico G, Vitagliano D, Morelli MP, Tuccillo C, Vecchione L, Capasso A, Orditura M, De Vita F et al (2010) Synergistic antitumor activity of sorafenib in combination with epidermal growth factor receptor inhibitors in colorectal and lung cancer cells. Clin Cancer Res 16(20):4990–5001
Mross K, Steinbild S, Baas F, Gmehling D, Radtke M, Voliotis D, Brendel E, Christensen O, Unger C (2007) Results from an in vitro and a clinical/pharmacological phase I study with the combination irinotecan and sorafenib. Eur J Cancer 43(1):55–63
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer National Cancer, Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Li L, Zhao M, Navid F, Pratz K, Smith BD, Rudek MA, Baker SD (2010) Quantitation of sorafenib and its active metabolite sorafenib N-oxide in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878(29):3033–3038
Zhao M, Rudek MA, He P, Hafner FT, Radtke M, Wright JJ, Smith BD, Messersmith WA, Hidalgo M, Baker SD (2007) A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci 846(1–2):1–7
Villarroel MC, Pratz KW, Xu L, Wright JJ, Smith BD, Rudek MA (2011) Plasma protein binding of sorafenib, a multi kinase inhibitor: in vitro and in cancer patients. Invest New Drugs
Jimeno A, Rudek MA, Purcell T, Laheru DA, Messersmith WA, Dancey J, Carducci MA, Baker SD, Hidalgo M, Donehower RC (2008) Phase I and pharmacokinetic study of UCN-01 in combination with irinotecan in patients with solid tumors. Cancer Chemother Pharmacol 61(3):423–433
Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, Stine A, Zhao M, Baker SD, Carducci MA, Wright JJ et al (2010) A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia 24(8):1437–1444
Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B (2007) Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 12(4):426–437
Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, Heym KM, Christensen R, Onciu M, Shurtleff SA, Pounds SB et al (2011) Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol 29(24):3293–3300
Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P (2006) Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol 57(5):685–692
van Erp NP, Baker SD, Zhao M, Rudek MA, Guchelaar HJ, Nortier JW, Sparreboom A, Gelderblom H (2005) Effect of milk thistle (Silybum marianum) on the pharmacokinetics of irinotecan. Clin Cancer Res 11(21):7800–7806
Kupsch P, Henning BF, Passarge K, Richly H, Wiesemann K, Hilger RA, Scheulen ME, Christensen O, Brendel E, Schwartz B, Hofstra E et al (2005) Results of a phase I trial of sorafenib (BAY 43–9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin Colorectal Cancer 5(3):188–196
Ychou M, Bouche O, Thezenas S, Francois E, Adenis A, Bennouna J, Taieb J, Desseigne F, Seitz J, Conroy T, Galais M et al (2011) Final results of a multicenter phase II trial assessing sorafenib (S) in combination with irinotecan (i) as second- or later-line treatment in metastatic colorectal cancer (mCRC) patients (pts) with KRAS-mutated tumors (mt; NEXIRI). ASCO Meeting Abstracts 29(15):e14002
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramirez J, Rudin CM, Vokes EE et al (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 22(8):1382–1388
Peer CJ, Sissung TM, Kim A, Jain L, Woo S, Gardner ER, Kirkland CT, Troutman SM, English BC, Richardson ED, Federspiel J et al. (2012) Sorafenib Is an Inhibitor of UGT1A1 but Is Metabolized by UGT1A9: Implications of Genetic Variants on Pharmacokinetics and Hyperbilirubinemia. Clinical cancer research: an official journal of the American Association for Cancer Research
Grothey A, Sobrero AF, Siena S, Falcone A, Ychou M, Lenz H-J, Yoshino T, Cihon F, Wagner A, Van Cutsem E, CORRECT Study Team (2012) Results of a phase III randomized, double-blind, placebo-controlled, multicenter trial (CORRECT) of regorafenib plus best supportive care (BSC) versus placebo plus BSC in patients (pts) with metastatic colorectal cancer (mCRC) who have progressed after standard therapies. ASCO Meeting Abstracts 30(4):LBA385
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D (2011) Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129(1):245–255
Acknowledgments
The authors would like to thank Sharyn Baker for helpful scientific discussions. The authors would also like to thank the patients and their families for participating in the study.
Disclosure of potential conflicts of interest
Dr. Messersmith has received commercial clinical research grant support from Bayer (major) via University of Colorado Cancer Center.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Funding
This research was supported by NIH/NCI grant 1K23CA115500 (WM), 1R21CA117125 (WM), U01 CA070095 (MC), and the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 RR025005). This publication was made possible by Grant Number UL1RR025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Nilofer Azad and Arvind Dasari contribute equally to the work presented.
Presented in part at the American Society of Clinical Oncology (ASCO) Annual Meeting, June 2008, Chicago, IL and ASCO Gastrointestinal Oncology Meeting, January 2011, San Francisco, CA
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.37 mb)
Rights and permissions
About this article
Cite this article
Azad, N., Dasari, A., Arcaroli, J. et al. Phase I pharmacokinetic and pharmacodynamic study of cetuximab, irinotecan and sorafenib in advanced colorectal cancer. Invest New Drugs 31, 345–354 (2013). https://doi.org/10.1007/s10637-012-9820-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9820-z