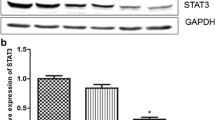
Abstract
Background
The inhibitor of apoptosis protein (IAP) family are reported to play important roles in cancer cells evading apoptosis. However, the significance of their expression in human esophageal squamous cell carcinoma (ESCC) cells remains uncertain.
Aims
The present study aimed to investigate the role of the IAP family members in tumor necrosis factor-α (TNF-α)-induced apoptosis of human ESCC cells.
Methods
Five human ESCC cell lines were pretreated with TNF-α, cycloheximide (CHX, protein synthesis inhibitor), epoxomicin (proteasome inhibitor). Apoptosis assay and protein study with Western blot testing were conducted. Knockdown experiments with IAP siRNA were conducted, and the effect on cell apoptosis was analyzed.
Results
Significant apoptosis was induced in five ESCC cell lines by TNF-α plus CHX stimulation, but not when treated with TNF-α or CHX alone. The protein expression levels of cIAP1 and XIAP were decreased by treatment with TNF-α in the presence of CHX, and the degree of cIAP1 and XIAP expression decrease was correlated with sensitivity to TNF-α plus CHX-induced apoptosis. Epoxomicin suppressed TNF-α plus CHX-induced degradation of survivin, cIAP1, and XIAP, in addition to apoptosis. A caspase inhibitor (z-VAD-fmk) suppressed TNF-α plus CHX-induced apoptosis, but did not suppress degradation of survivin, cIAP1, and XIAP. Furthermore, cIAP1 or XIAP siRNA transfected cells underwent apoptosis in response to treatment with TNF-α alone. Double knockdown of both genes resulted in further increased apoptosis.
Conclusion
cIAP1 and XIAP play an essential role in the resistance of ESCC cells against apoptosis.





Similar content being viewed by others
Abbreviations
- CHX:
-
Cycloheximide
- cIAP1:
-
Cellular IAP
- FBS:
-
Fetal bovine serum
- IAP:
-
Inhibitor of apoptosis
- PI:
-
Propidium iodide
- TNF-α:
-
Tumor necrosis factor-α
- XIAP:
-
X-linked IAP
References
Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190.
Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703.
Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635.
Miller LK. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol. 1999;9:323–328.
Thao Y, Conze DB, Hanover JA, Ashwell JD. Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:7777–7782.
Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004;14:231–243.
Tamm I, Kornblau SM, Segall H, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803.
Kempkensteffen C, Hinz S, Christoph F, et al. Expression parameters of the inhibitors of apoptosis cIAP1 and cIAP2 in renal cell carcinomas and their prognostic relevance. Int J Cancer. 2006;120:1081–1086.
Zhu H, Wang Q, Hu C, et al. High expression of survivin predicts poor prognosis in esophageal squamous cell carcinoma following radiotherapy. Tumor Biol. 2011;32:1147–1153.
Imoto I, Tsuda H, Hirasawa A, et al. Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res. 2002;62:4860–4866.
Hasegawa T, Suzuki K, Sakamoto C, et al. Expression of the inhibitor of apoptosis (IAP) family members in human neutrophils: up-regulation of cIAP2 by granulocyte colony-stimulating factor and overexpression of cIAP2 in chronic neutrophilic leukemia. Blood. 2003;101:1164–1171.
Liu Z, Sun C, Olejniczak ET, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008.
Vince JE, Wong WW, Khan N, et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell. 2007;131:682–693.
Darding M, Meier P. IAPs: guardians of RIPK1. Cell Death Differ. 2012;19:58–66.
Kitagawa M, Shiozaki A, Ichikawa D, et al. Tumor necrosis factor-α-induced apoptosis of gastric cancer MKN28 cells: accelerated degradation of the inhibitor of apoptosis family members. Arch Biochem Biophys. 2015;566:43–48.
Tanimura Y, Kokuryo T, Tsunoda N, et al. Tumor necrosis factor alpha promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett. 2005;219:205–213.
Shiozaki A, Shimizu H, Ichikawa D, et al. Claudin 1 mediates tumor necrosis factor alpha-induced cell migration in human gastric cancer cells. World J Gastroenterol. 2014;20:17863–17876.
Wu ST, Sun GH, Hsu CY, et al. Tumor necrosis factor-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a nuclear factor kappa B-independent mechanism. Exp Biol Med (Maywood). 2011;236:1022–1029.
Huang YJ, Qi WX, He AN, Sun YJ, Shen Z, Yao Y. The prognostic value of survivin expression in patients with colorectal carcinoma: a meta-analysis. Jpn J Clin Oncol. 2013;43:988–995.
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (C) (26461988) and Grants-in-Aid for Young Scientists (B) (15K19903) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Shoichiro Hikami, Atsushi Shiozaki and Maki Kitagawa-Juge contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hikami, S., Shiozaki, A., Kitagawa-Juge, M. et al. The Role of cIAP1 and XIAP in Apoptosis Induced by Tumor Necrosis Factor Alpha in Esophageal Squamous Cell Carcinoma Cells. Dig Dis Sci 62, 652–659 (2017). https://doi.org/10.1007/s10620-016-4430-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4430-9