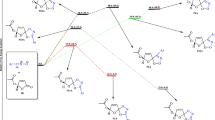
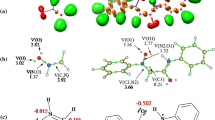
Using DFT/B3LYP and ab initio RHF quantum-chemical calculations in the triple-zeta basis set 6-31++G** the endo and exo cycloaddition mechanism for the interaction of ethyl vinyl ether, trimethylsilyloxybutadiene, or cyclopentadiene with 5-nitro-7,8-furoxanoquinolines was studied in details. Considering that both in solutions and crystals nitrofuroxanoquinoline exists as an inseparable mixture of two N-oxide tautomers, all cycloaddition processes were studied for both of them. The studied mechanisms practically do not depend on the location of the exocyclic oxygen atom in nitrofuroxanoquinoline molecule. At the first step of all reactions the conjugated nitroarene fragments C=C–N=O react with nucleophilic reagents following the mechanism of endo-[4+2] cycloaddition with inverse electronic demand. Further (in the cases of trimethylsilyloxybutadiene and cyclopentadiene) endo-[4+2] cycloadducts recyclize spontaneously according to the mechanism of [3,3] sigmatropic rearrangement into more thermodynamically stable, experimentally detected endo-[2+4] cycloadducts. Both endo-[4+2] and endo-[2+4] cycloadducts obtained from cyclopentadiene and nitrofuroxanoquinoline have been experimentally isolated and characterized. For this case, the kinetic and activation parameters of [4+2] → [2+4] transformation have been studied by 1H NMR method, which have shown an excellent agreement with quantum-chemical results. In all cases the exo processes are one-step reactions, less favorable kinetically than their endo competitors.














Similar content being viewed by others
References
(a) Bunnett, J. F.; Zahler, R. E. Chem. Rev. 1951, 49, 273. (b) Miller, J. Aromatic Nucleophilic Substitution; Elsevier: Amsterdam, 1968. (c) Buncel, E.; Crampton, M. R.; Strauss, M. J.; Terrier, F. Electron-Deficient Aromatic- and Heteroaromatic Base Interactions; Elsevier: Amsterdam, 1984. (d) Terrier, F. Nucleophilic Aromatic Displacement. The Influence of the Nitro Group; VCH: New York, 1991.
(a) Beck, J. R. Tetrahedron, 1978, 34, 2057. (b) Vlasov, V. M. Russ. Chem. Rev. 2003, 72, 681. [Usp. Khim. 2003, 72, 764.] (c) Forlani, L. In The Chemistry of Amino, Nitroso, Nitro and Related Groups; Patai, S., Ed.; John Wiley and Sons: Chichester, 1996, p. 423.
(a) Terrier, F. Chem. Rev. 1982, 82, 77. (b) Buncel, E.; Dust, J. M.; Terrier, F. Chem. Rev. 1995, 95, 2261. (c) Strauss, M. J. Chem. Rev. 1970, 70, 667. (d) Artamkina, G. A.; Egorov, M. P.; Beletskaya, I. P. Chem. Rev. 1982, 82, 427.
(a) Terrier, F.; Millot F.; Norris, W. P. J. Am. Chem. Soc. 1976, 98, 5883. (b) Terrier, F.; Kizilian, E.; Halle, J. C.; Buncel, E. J. Am. Chem. Soc. 1992, 114, 1740. (c) Di Nunno, L.; Florio, S.; Todesco, P. E. J. Chem. Soc., Perkin Trans. 2 1975, 1469. (d) Read R. W.; Norris, W. P. Aust. J. Chem. 1985, 38, 435. (e) Vichard, D.; Boubaker, T.; Terrier, F.; Pouet, M. J.; Dust, J. M.; Buncel, E. Can. J. Chem. 2001, 79, 1617. (f) Boubaker, T.; Chatrousse, A. P.; Terrier, F.; Tangour, B.; Dust, J. M.; Buncel, E. J. Chem. Soc., Perkin Trans. 2 2002, 1627. (g) Boubaker, T.; Goumont, R.; Jan, E.; Terrier, F. Org. Biomol. Chem. 2003, 1, 2764. (h) Filatov, I. E.; Rusinov, G. L.; Chupakhin, O. N.; Solans, X.; Font-Bardia, M.; Font-Altaba, M. Russ. Chem. Bull. 1994, 43, 1214. [Izv. Akad. Nauk, Ser. Khim. 1994, 1278.] (i) Strauss, M. J.; Renfrow, R. A.; Buncel, E. J. Am. Chem. Soc. 1983, 105, 2473. (j) Buncel, E.; Renfrow, R. A.; Strauss, M. J. J. Org. Chem., 1987, 52, 488. (k) Crampton, M. R.; Rabbitt, L. C.; Terrier, F. Can. J. Chem. 1999, 77, 639. (l) Crampton, M. R.; Lunn, R. E. A.; Lucas, D. Org. Biomol. Chem. 2003, 1, 3438. (m) Buncel, E.; Dust, J. M.; Manderville, R. A. J. Am. Chem. Soc. 1996, 118, 6072. (n) Terrier, F.; Sebban, M.; Goumont, R.; Hallé, J. C.; Moutiers, G.; Cangelosi, I.; Buncel, E. J. Org. Chem. 2000, 65, 7391. (o) Sebban, M.; Goumont, R.; Hallé, J. C.; Terrier, F.; Marrot, J. Chem. Commun. 1999, 1009. (p) Boga, C.; Forlani, L.; Del Vecchio, E.; Mazzanti, A.; Todesco, P. E. Angew. Chem., Int. Ed. 2005, 44, 3285. (q) Forlani, L.; Tocke, A. L.; Del Vecchio, E.; Lakhdar, S.; Goumont, R.; Terrier, F. J. Org. Chem. 2006, 71, 5527. (r) Boga, C.; Del Vecchio, E.; Forlani, L.; Goumont, R.; Terrier, F.; Tozzi, S. Chem.–Eur. J. 2007, 13, 9600.
(a) Terrier, F.; Lakhdar, S.; Boubaker, T.; Goumont, R. J. Org. Chem. 2005, 70, 6242. (b) Lakhdar, S.; Goumont, R.; Boubaker, T.; Mokhtari, M.; Terrier, F. Org. Biomol. Chem. 2006, 4, 1910. (c) Lakhdar, S.; Goumont, R.; Terrier, F.; Boubaker, T.; Dust, J. M.; Buncel, E. Org. Biomol. Chem. 2007, 5, 1744. (d) Terrier, F.; Xiao, L.; Hlaibi, M.; Halle, J. C. J. Chem. Soc., Perkin Trans. 2, 1993, 337. (e) Rodriguez- Dafonte, P.; Terrier, F.; Lakhdar, S.; Kurbatov, S.; Goumont, R. J. Org. Chem. 2009, 74, 3305. (f) Crampton, M. R.; Delaney, J.; Rabbitt, L. C. J. Chem. Soc., Perkin Trans. 2, 1999, 2473. (g) Boga, C.; Del Vecchio, E.; Forlani, L.; Mazzanti, A.; Lario, C. M.; Todesco, P. E.; Tozzi, S. J. Org. Chem. 2009, 74, 5568. (h) Jin, P.; Li, F.; Riley, K.; Lenoir, D.; Schleyer, P. v. R.; Chen, Z. J. Org. Chem. 2010, 75, 3761. (i) Kurbatov, S.; Tatarov, A.; Minkin, V.; Goumont, R.; Terrier, F. Chem. Commun. 2006, 4279. (j) Tatarov, A.; Kurbatov, S.; Borodkin, G.; Goumont, R.; Terrier, F. Tetrahedron 2010, 66, 995.
(a) Hallé, J. C.; Vichard, D.; Pouet, M. J.; Terrier, F. J. Org. Chem. 1997, 62, 7178. (b) Vichard, D.; Hallé, J.-C.; Huguet, B.; Pouet, M.-J.; Riou, D.; Terrier, F. Chem. Commun. 1998, 791. (c) Kresze, G.; Bathelt, H. Tetrahedron, 1973, 29, 1043. (d) Sepulcri, P.; Halle, J.C.; Goumont, R.; Riou, D.; Terrier, F. J. Org. Chem. 1999, 64, 9254. (e) Goumont, R.; Sebban, M.; Sépulcri, P.; Marrot, J.; Terrier, F. Tetrahedron 2002, 58, 3249. (f) Pugnaud, A.; Masure, D.; Hallé, J. C.; Chaquin, P. J. Org. Chem. 1997, 62, 8687. (g) Sepulcri, P.; Goumont, R.; Hallé, J. C.; Riou, D.; Terrier, F. J. Chem. Soc., Perkin Trans. 2 2000, 51. (h) Goumont, R.; Sebban, M.; Terrier, F. Chem. Commun. 2002, 2110. (i) Vichard, D.; Alvey, L.; Terrier, F. Tetrahedron Lett. 2001, 42, 7571. (j) Steglenko, D. V.; Kletsky, M. E.; Kurbatov, S. V.; Tatarov, A. V.; Minkin, V. I.; Goumont, R.; Terrier, F. J. Phys. Org. Chem. 2009, 22, 298. (k) Kurbatov, S.; Goumont, R.; Lakhdar, S.; Marrot, J.; Terrier, F. Tetrahedron 2005, 61, 8167. (l) Lakhdar, S.; Terrier, F.; Vichard, D.; Berionni, G.; El Guesmi, N.; Goumont, R.; Boubaker, T. Chem.–Eur. J. 2010, 16, 5681.
(a) Buncel, E.; Terrier, F. Org. Biomol. Chem. 2010, 8, 2285. (b) Terrier, F.; Dust, J. M.; Buncel, E. Tetrahedron 2012, 68, 1829.
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R. Tetrahedron 2002, 58, 4417.
Pérez, P.; Domingo, L. R.; Aurell, M. J.; Contreras, R. Tetrahedron 2003, 59, 3117.
Parr, R. G.; von Szentpály, L.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922.
(a) Bastrakov, M. A.; Starosotnikov, A. M.; Fedyanin, I. V.; Kachala, V. V.; Shevelev, S. A. Mendeleev Commun. 2014, 24, 203. (b) Bastrakov, M. A.; Starosotnikov, A. M.; Kachala, V. V.; Fedyanin, I. V.; Shevelev, S. A. Asian J. Org. Chem. 2015, 4, 146.
Nösberger, P.; Bauder, A.; Günthard, H. H. Chem. Phys. 1973, 1, 426.
(a) Sepulcri, P.; Hallé, J. C.; Goumont, R.; Riou, D.; Terrier, F. J. Org. Chem. 1999, 64, 9254. (b) Hallé, J. C.; Vichard, D.; Pouet, M. J.; Terrier, F. J. Org. Chem. 1997, 62, 7178.
Kurbatov, S. V.; Goumont, R.; Marrot, J.; Terrier, F. Tetrahedron Lett. 2004, 45, 1037.
Pugnaud, S.; Masure, D.; Hallé, J.-C.; Chaquin, P. J. Org. Chem. 1997, 62, 8687.
Steglenko, D. V. Ph. D. Thesis, Rostov-on-Don, 2012.
(a) Becke, A. D. J. Chem. Phys. 1993, 98, 5648. (b) Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785.
Hehre, W. J.; Radom, L.; Schleyer, P. v. R.; Pople, J. A. Ab initio Molecular Orbital Theory, Wiley – Interscience: New York, 1986.
Li, X.; Frisch, M. J. J. Chem. Theory Comput. 2006, 2, 835.
(a) Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735. (b) Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. B 1988, 88, 899.
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R. J. Phys. Chem. A 2002, 106, 6871.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, 2009.
Fuentealba, P.; Pérez, P.; Contreras, R. J. Chem. Phys. 2000, 113, 2544.
Arroyo, P.; Picher, M. T.; Domingo, L. R.; Terrier, F. Tetrahedron 2005, 61, 7359.
Yamabe, S.; Minato, T. J. Org. Chem. 2000, 65, 1830.
Craig, D.; Shipman, J. J.; Fowler, R. B. J. Am. Chem. Soc. 1961, 83, 2885.
Gleiter, R.; Bohm, M. C. Pure Appl. Chem. 1983, 55, 237.
(a) Hine, J. J. Org. Chem. 1966, 31, 1236. (b) Hine, J. In Advances in Physical Organic Chemistry; Gold, V.; Bethell, D., Eds.; Academic Press: London, 1977, vol. 15, p. 1. (c) Igawa, A.; Fukutome, H. Chem. Phys. Lett. 1987, 133, 399.
Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley-VCH: Weinheim, 2013, p. 163.
The work was performed with the financial support of the Russian Science Fund (project 14-13-00103).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(9), 845–857
Rights and permissions
About this article
Cite this article
Steglenko, D.V., Shevelev, S.A., Kletskii, M.E. et al. Quantum-chemical and NMR study of nitrofuroxanoquinoline cycloaddition. Chem Heterocycl Comp 51, 845–857 (2015). https://doi.org/10.1007/s10593-015-1785-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1785-3