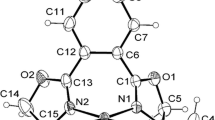
Potential atropisomeric ligands, diphenylphosphane derivatives of bis-triazoles, were synthesized by direct phosphanylation of the starting bis-triazoles with chlorodiphenylphosphane. Their crystal structure was elucidated by X-ray diffractional analysis. The conformations of these compounds were modeled by DFT calculations.




Similar content being viewed by others
References
G. Bringmann, A. Wuzik, M. Breuning, P. Henschel, K. Peters, and E.-M. Peters, Tetrahedron: Asymmetry, 10, 3025 (1999).
T. Shimada, A. Kina, and T. Hayashi, J. Org. Chem., 68, 6329 (2003).
G. Bringmann, A. J. Price Mortimer, P. A. Keller, M. J. Gresser, J. Garner, and M. Breuning, Angew. Chem., Int. Ed., 44, 5384 (2005).
A. Miyashita, A. Yasuda, H. Takaya, K. Toriumi, T. Ito, T. Souchi, and R. Noyori, J. Am. Chem. Soc., 102,7932 (1980).
M. Berthod, G. Mignani, G. Woodward, and M. Lemaire, Chem. Rev., 105, 1801 (2005).
H. Kumobayashi, Recl. Trav. Chim. Pays-Bas, 115, 201 (1996).
I. Alkorta, J. Elguero, C. Roussel, N. Vanthuyne, and P. Piras, in: A. Katritzky (editor), Advances in Heterocyclic Chemistry, Vol. 105, Elsevier, New York (2012), p. 1.
G. Celentano, T. Benincori, S. Radaelli, M. Sada, and F. Sannicolò, J. Organomet. Chem., 643–644, 424 (2002).
T. Benincori, E. Brenna, F. Sannicolò, L. Trimarco, P. Antognazza, E. Cesarotti, F. Demartin, and T. Pilati, J. Org. Chem., 61, 6244 (1996).
R. K. Bartlett and I. R. Humphrey, J. Chem. Soc. C, 1664 (1967).
A. Pollak and M. Tišler, Tetrahedron, 22, 2073 (1966).
I. B. Lundina, L. E. Deev, E. G. Kovalev, and I. Ya. Postovskii, Chem. Heterocycl. Compd., 9, 265 (1973). [Khim. Geterotsikl. Soedin., 285 (1973)].
E. V. Zarudnitskii, V. V. Ivanov, A. A. Yurchenko, A. M. Pinchuk, and A. A. Tolmachev, Heteroat. Chem., 13, 146 (2002).
A. A. Tolmachev, E. V. Zarudnitskii, S. I. Dovgopoly, A. O. Pushechnikov, A. A. Yurchenko, and A. M. Pinchuk, Chem. Heterocycl. Compd., 35, 1249 (1999). [Khim. Geterotsikl. Soedin., 1432 (1999)].
A. P. Marchenko, H. N. Koidan, E. V. Zarudnitskii, A. N. Hurieva, A. A. Kirilchuk, A. A. Yurchenko, A. Biffis, and A. N. Kostyuk, Organometallics, 31, 8257 (2012).
A. D. Becke, Phys. Rev. A, 38, 3098 (1988).
J. P. Perdew, Phys. Rev. B, 33, 8822 (1986).
S. Grimme, J. Antony, T. Schwabe, and C. Mück-Lichtenfeld, Org. Biomol. Chem., 5, 741 (2007).
M. D. Jones, F. A. Almeida Paz, J. E. Davies, and B. F. G. Johnson, Acta Crystallogr., Sect. E: Struct. Rep. Online, E59, o535 (2003).
F. Furche, R. Ahlrichs, C. Hättig, W. Klopper, M. Sierka, and F. Weigend, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 4, 91 (2014).
R. Ahlrichs, M. Bär, M. Häser, H. Horn, and C. Kölmel, Chem. Phys. Lett., 162, 165 (1989).
A. Schäfer, C. Huber, and R. Ahlrichs, J. Chem. Phys., 100, 5829 (1994).
The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. M. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukudal, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian-03, Revision C.02, Gaussian, Inc., Pittsburgh (2003).
G. M. Sheldrick, SHELXS97. Program for the Solution of Crystal Structure, University of Göttingen (1997).
G. M. Sheldrick, SHELXL97. Program for the Refinement of crystal Structures, University of Göttingen (1997).
D. J. Watkin, C. K. Prout, J. R. Carruthers, and P. W. Betteridge, CRYSTALS Issue 10, Chemical Crystallography Laboratory, Oxford (1996).
The authors are grateful to Prof. Uwe Manthe and Dr. Thorsten Tönsing of Bielefeld University, Germany, for providing access to computer cluster and GAUSSIAN-03 and TURBOMOLE software, as well as for technical support of calculations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1697-1705, November, 2014.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 627 kb)
Rights and permissions
About this article
Cite this article
Kirilchuk, A.A., Yurchenko, A.A., Vlasenko, Y.G. et al. Synthesis and Structure of Phosphanylated Bis-Triazoles as Potential Ligands for Chiral Catalysts. Chem Heterocycl Comp 50, 1559–1566 (2015). https://doi.org/10.1007/s10593-014-1624-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1624-y