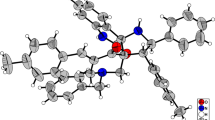
This is the first report of the use of asymmetrical cross-conjugated unsaturated ketones as dipolarophiles in 1,3-dipolar cycloaddition reactions. These reactions proceed without chemoselectivity at the two nonequivalent reaction sites of the dienone but diastereoselectively to give a mixture of structural isomers of endo-cycloaddition products. Monoenones were used to synthesize spiropyrrolidizines with high diastereoselectivity.



Similar content being viewed by others
References
A. Padwa (editor), 1,3-Dipolar Cycloaddition Chemistry , Vols. 1-2, Wiley-Interscience, New York (1984).
I. Coldham and R. Hufton, Chem. Rev., 105, 2765 (2005).
N. Arumugam, G. Periyasami, R. Raghunathan, S. Kamalraj, and J. Muthumary, Eur. J. Med. Chem., 46, 600 (2011).
R. Prasanna, S. Purushothaman, and R. Raghunathan, Tetrahedron Lett., 51, 4538 (2010).
H. Pellissier, Tetrahedron, 63, 3235 (2007).
R. Grigg, F. Heaney, J. Idle, and A. Somasunderam, Tetrahedron Lett., 31, 2767 (1990).
V. Padmavathi, K. V. Reddy, A. Padmaja, and D. Bhaskar Reddy, Synth. Commun., 32, 1227 (2002).
R. S. Kumar, S. M. Rajesh, S. Perumal, Y. Yogeeswari, and D. Sriram, Tetrahedron Asymmetry, 21, 1315 (2010).
R. R. Kumar and S. Perumal, Tetrahedron, 63, 7850 (2007).
G. Sridhar, T. Gunasundari, and R. Raghunathan, Tetrahedron Lett., 48, 319 (2007).
H. A. Dondas, C. W. G. Fishwick, R. Grigg, and C. Kilner, Tetrahedron, 60, 3473 (2004).
M. J. Taghizadeh, H. Arvinnezhad, S. Samadi, K. Jadidi, A. Javidan, and B. Notash, Tetrahedron Lett., 53, 5148 (2012).
A. Amal Raj and R. Raghunathan, Tetrahedron, 57, 10293 (2001).
J. Jayashankaran, R. D. R. S. Manian, R. Venkatesan, and R. Raghunathan, Tetrahedron, 61, 5595 (2005).
N. Boukamcha, R. Gharbi, M.-T. Martin, A. Chiaroni, Z. Mighri, and F. A. Khemiss, Tetrahedron, 55, 449 (1999).
N. V. Lakshmi, P. Thirumurugan, and P. T. Perumal, Tetrahedron Lett., 51, 1064 (2010).
I. N. Klochkova, A. A. Anis'kov, and M. P. Shchekina, Khim. Geterotsikl. Soedin., 1425 (2011). [Chem. Heterocycl. Compd., 47, 1176 (2011)].
M. F. Aly, R. Grigg, S. Thianpatanagul, and V. Sridharan, J. Chem. Soc., Perkin Trans. 1, 949 (1988).
R. Grigg, J. Idle, P. McMeekin, and D. Vipond, J. Chem. Soc., Chem. Commun., 49 (1987).
R. Grigg, S. Surendrakumar, S. Thianpatanagul, and D. Vipond, J. Chem. Soc., Perkin Trans. 1, 2693 (1988).
R. Sustmann, Pure Appl. Chem., 40, 569 (1974).
M. L. Kuznetsov, Usp. Khim., 75, 1045 (2006). [Russ. Chem. Rev., 75, 935 (2006)].
Y. Sarrafi, M. Hamzehloueian, K. Alimohammadi, and S. Yeganegi, J. Mol. Struct., 1030, 168 (2012).
K. Alimohammadi, Y. Sarrafi, M. Tajbakhsh, S. Yeganegi, and M. Hamzehloueian, Tetrahedron, 67, 1589 (2011).
G. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., A64, 112 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 527-536, April, 2014.
Rights and permissions
About this article
Cite this article
Klochkova, I.N., Shchekina, M.P. & Anis’kov, A.A. Synthesis of Spiropyrrolidines and Spiropyrrolizidines from Azomethine Ylides. Chem Heterocycl Comp 50, 479–488 (2014). https://doi.org/10.1007/s10593-014-1498-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1498-z