Abstract
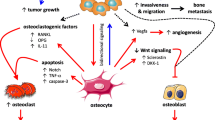
Anti-resorptive bisphosphonates (BPs) have been clinically used to prevent cancer-bone metastasis and cancer-induced bone pathologies despite the fact that the phenotypic response of the cancer-bone interactions to BP exposure is “uncharted territory”. This study offers unique insights into the interplay between cancer stem cells and osteocytes/osteoblasts and mesenchymal stem cells using a three-dimensional (3D) live cancer-bone interactive model. We provide extraordinary cryptic details of the biological events that occur as a result of alendronate (ALN) treatment using 3D live cancer-bone model systems under specific bone remodeling stages. While cancer cells are susceptible to BP treatment in the absence of bone, they are totally unaffected in the presence of bone. Cancer cells colonize live bone irrespective of whether the bone is committed to bone resorption or formation and hence, cancer-bone metastasis/interactions are though to be “independent of bone remodeling stages”. In our 3D live bone model systems, ALN inhibited bone resorption at the osteoclast differentiation level through effects of mineral-bound ALN on osteocytes and osteoblasts. The mineral-bound ALN rendered bone incapable of osteoblast differentiation, while cancer cells colonize the bone with striking morphological adaptations which led to a conclusion that a direct anti-cancer effect of BPs in a “live or in vivo” bone microenvironment is implausible. The above studies were complemented with mass spectrometric analysis of the media from cancer-bone organ cultures in the absence and presence of ALN. The mineral-bound ALN impacts the bone organs by limiting transformation of mesenchymal stem cells to osteoblasts and leads to diminished endosteal cell population and degenerated osteocytes within the mineralized bone matrix.












Similar content being viewed by others
References
Giger EV, Castagner B, Leroux JC (2013) Biomedical applications of bisphosphonates. J Control Release 167:175–188
Hillner BE et al (2000) American society of clinical oncology guideline on the role of bisphosphonates in breast cancer. J Clin Oncol 18:1378–1391
Clézardin P, Ebetino FH, Fournier PGJ (2005) Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res 65:4971–4974
Daubine F, Le Gall C, Gasser J, Green J, Clezardin P (2007) Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst 99:322e–330e
Green J, Clezardin P (2010) The molecular basis of bisphosphonate activity: a preclinical perspective. Semin Oncol 37(Suppl. 1):S3–S11
Clezardin P (2011) Bisphosphonates’ antitumor activity: an unravelled side of a multifaceted drug class. Bone 48:71–79
Costa L, Harper P, Coleman RE, Lipton A (2011) Anticancer evidence for zoledronic acid across the cancer continuum. Crit Rev Oncol Hematol 77(suppl 1):S31–S37
Aft R et al (2010) Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol 11:421–428
Coleman RE (2008) Risks and benefits of bisphosphonates. Br J Cancer 98:1736–1740
Meads MB, Hazlehurst LA, Dalton WS (2008) The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 14:2519–2526
Holen I, Coleman RE (2010) Anti-tumour activity of bisphosphonates in preclinical models of breast cancer. Breast Cancer Res 12(6):214–227
Rack B et al (2010) Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res 30:1807–1813
Wilson C, Coleman RE (2012) Seed, soil and secreted hormones: potential interactions of breast cancer cells with their endocrine/paracrine microenvironment and implications for treatment with bisphosphonates. Cancer Treat Rev 38(7):877–889
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Brown JE, Neville-Webbe H, Coleman RE (2004) The role of bisphosphonates in breast and prostate cancers. Endocr Relat Cancer 11:207–224
Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, Munshi NC, Anderson KC (2007) The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am 21:1007–1034
Zipori D (2010) The hemopoietic stem cell niche versus the microenvironment of the multiple myeloma- tumor initiating cell. Cancer Microenviron 3(1):15–28
Gnant M et al (2009) Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Eng J Med 360(7):679–691
Morgan GJ et al (2010) First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 376:1989–1999
Smith MR et al (2012) Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379:39–46
Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS (2012) Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med 18:1359–1368
Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J (1997) Cell-cycle arrest versus cell death in cancer therapy. Nat Med 3:1034–1036
Selander KS, Monkkonen J, Karhukorpi EK, Harkonen P, Hannuniemi R, Vaananen HK (1996) Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol Pharmacol 5:1127–1138
Murakami H, Takahashi N, Sasaki T, Udagawa N, Tanaka S, Nakamura I, Zhang D, Barbier A, Suda T (1995) A possible mechanism of the specific action of bisphosphonates on osteoclasts: tiludronate preferentially affects polarized osteoclasts having ruffled borders. Bone 17(2):137–144
Hughes DE, MacDonald BR, Russell RG, Gowen M (1989) Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest 83:1930–1935
Vescovi P, Merigo E, Meleti M, Manfredi M, Guidotti R, Nammour S (2012) Bisphosphonates-related osteonecrosis of the jaws: a concise review of the literature and a report of a single-centre experience with 151 patients. J Oral Pathol Med 41:214–221
Giusti A, Hamdy NA, Papapoulos SE (2010) Atypical fractures of the femur and bisphosphonate therapy A systematic review of case/case series studies. Bone 47:169–180
Obias JH, Chow JW, Chambers TJ (1993) 3-Amino-1-hydroxypropylidine-1-bisphosphonate (AHPrBP) suppresses not only the induction of new, but also the persistence of existing bone-forming surfaces in rat cancellous bone. Bone 14:619–623
Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G (1998) Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 22:455–461
Idris AI, Rojas J, Greig IR, Van’t Hof RJ, Ralston SH (2008) Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int 82:191–201
Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS (2004) Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 25:4105–4115
Iwata K, Li J, Follet H, Phipps RJ, Burr DB (2006) Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone 39:1053–1058
Duque G, Rivas D (2007) Alendronate has an anabolic effect on bone through the differentiation of mesenchymal stem cells. Bone Miner Res 22:1603–1611
Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM, Clézardin P (2000) Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 60:2949–2954
Gnant M, Clezardin P (2012) Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev 38:407–415
Coleman R, Gnant M, Morgan G, Clezardin P (2012) Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst 104:1059–1067
Hue TF, Cummings SR, Cauley JA, Bauer DC, Ensrud KE, Barrett-Connor E, Black DM (2014) Effect of bisphosphonate use on risk of postmenopausal breast cancer results from the randomized clinical trials of alendronate and zoledronic acid. JAMA Intern Med 174:1550–1557
Liu Y, Zhao S, Chen W, Hu F, Zhu L, Zhang Q, Zhao Y (2012) Bisphosphonate use and the risk of breast cancer: a meta-analysis of published literature. Clin Breast Cancer 12:276–281
Coleman R et al (2013) Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: a meta-analysis of individual patient data from randomized trials. In: San Antonio breast cancer symposium, San Antonio, TX (Abstract S4-07)
Curtin P, Youm H, Salih E (2012) Three-dimensional cancer-bone metastasis model using ex vivo co-cultures of live calvarial bones and cancer cells. Biomaterials 33:1065–1078
Liu J, Czernick D, Lin S-C, Alasmari A, Dibart S, Salih E (2013) Novel bioactivity of phosvitin in connective tissue and bone organogenesis revealed by live calvarial bone organ culture models. Dev Biol 381:256–275
Simonet WS et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, Fan M, Jun S (2008) randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 26(30):4875–4882
Shiozawa Y et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121:1298–1312
Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, Smid M, Foekens JA, Massagué J (2013) Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154:1060–1073
Wang N, Docherty FE, Brown HK, Reeves KJ, Fowles AC, Ottewell PD, Dear TN, Holen I, Croucher PI, Eaton CL (2014) Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis—evidence from in vivo models. J Bone Miner Res 29:2688–2696
Wang H et al (2015) The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27:193–210
van der Pluijm G, Que I, Sijmons B, Buijs JT, Löwik CW, Wetterwald A, Thalmann GN, Papapoulos SE, Cecchini MG (2005) Interference with the microenvironmental support impairs the de novo formation of bone metastases in vivo. Cancer Res 65:7682–7690
Diel IJ, Jaschke A, Solomayer EF, Gollan C, Bastert G, Sohn C, Schuetz F (2008) Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol 19:2007–2011
Saarto T, Blomqvist C, Virkkunen P, Elomaa I (2001) Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol 19:10–17
Saarto T, Vehmanen L, Virkkunen P, Blomqvist C (2004) Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol 43:650–656
Powles T, Paterson A, McCloskey E, Schein P, Scheffler B, Tidy A, Ashley S, Smith I, Ottestad L, Kanis J (2006) Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res 8:R13
Palaska PK, Cartsos V, Zavras AI (2009) Bisphosphonates and time to osteonecrosis development. Oncologist 14:1154–1166
Thiery JP (2002) Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer 2:442–454
Brehmer B, Biesterfeld S, Jakse G (2003) Expression of matrix metalloproteinases (MMP-2 and -9) and their inhibitors (TIMP-1 and -2) in prostate cancer tissue. Prostate Cancer Prostatic Dis 6:217–222
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ (1997) Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861–1872
Radisky DC et al (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436:123–127
Acknowledgments
The work was made possible by private funds of Dr. Salih, and support from The Department of Periodontology, Goldman School of Dental Medicine, Boston University and was not supported by NIH/NIDCR funds. The authors thank Professor M. Kirber for use of the LSM 710 2-photon confocal microscope in the BUMC “Cellular Imaging Core Facility”; and Professor J. Pudney (Department of Obstetrics and Gynecology) and Professor Steven Borkan (Department of Medicine), Boston University School of Medicine for pre-submission review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Abeer Alasmari and Erdjan Salih have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alasmari, A., Lin, SC., Dibart, S. et al. Bone microenvironment-mediated resistance of cancer cells to bisphosphonates and impact on bone osteocytes/stem cells. Clin Exp Metastasis 33, 563–588 (2016). https://doi.org/10.1007/s10585-016-9798-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-016-9798-6