Abstract
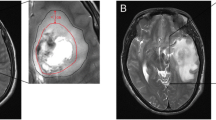
To analyze the prognostic value of the extent of peritumoral brain edema in patients operated for single brain metastases (BM), we retrospectively evaluated pre-operative magnetic resonance images in a discovery cohort of 129 patients and a validation cohort of 118 patients, who underwent neurosurgical resection of a single BM in two different hospitals. We recorded clinical parameters and immunohistochemically assessed the Ki67 index, the microvascularization patterns and the expression of hypoxia-induced factor 1 alpha (HIF1a) in the BM tissue specimens retrieved at neurosurgery. Statistical analysis including uni- and multivariate survival analyses were performed. Baseline characteristics were well balanced between the discovery and validation cohorts. In univariate analysis, we found a significant association of favorable overall survival time with young patient age, high Karnofsky performance score, low graded prognostic assessment (GPA) class, absence of extracranial metastases, adjuvant treatment with whole brain radiotherapy and, surprisingly, large brain edema. In multivariate analysis, only GPA and extent of brain edema remained independent prognostic parameters. The prognostic impact of the extent of brain edema was consistent in the two patient cohorts. Furthermore, we found a significant correlation of small brain edema with brain-invasive tumor growth pattern as assessed intraoperatively by the neurosurgeon, low neo-angiogenic activity and low expression of HIF1a. Extent of brain edema independently correlates with prognosis in patients operated for single BM. In conclusion, patients with small peritumoral edema have shorter survival times and their tumors are characterized by a more brain-invasive growth, lower HIF1a expression and less angiogenic activity.


Similar content being viewed by others
Abbreviations
- BM:
-
Brain metastases
- HIF1a:
-
Hypoxia-induced factor 1 alpha
- GPA:
-
Graded prognostic assessment
- OS:
-
Overall survival
- NSCLC:
-
Non-small cell lung cancer
- SCLC:
-
Small cell lung cancer
- HER2:
-
In human epidermal growth factor receptor 2
- BMFS:
-
Brain metastasis free survival
- MR:
-
Magnetic resonance
- KPS:
-
Karnofsky performance score
- WBRT:
-
Whole-brain radiation therapy
- CNS:
-
Central nervous system
References
Walker AE, Robins M, Weinfeld FD (1985) Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology 35(2):219–226
Percy AK, Elveback LR, Okazaki H, Kurland LT (1972) Neoplasms of the central nervous system. Epidemiologic considerations. Neurology 22(1):40–48
Smedby KE, Brandt L, Backlund ML, Blomqvist P (2009) Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer 101(11):1919–1924
Schouten LJ, Rutten J, Huveneers HA, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94(10):2698–2705
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973–2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22(14):2865–2872
Barnholtz-Sloan JS, Yu C, Sloan AE, Vengoechea J, Wang M, Dignam JJ, Vogelbaum MA, Sperduto PW, Mehta MP, Machtay M, Kattan MW (2012) A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol 14(7):910
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30(4):419–425
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70(2):510–514
Wohrer A, Waldhor T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mosenbacher U, Kiefer A, Maier H, Motz R, Reiner-Concin A, Richling B, Idriceanu C, Scarpatetti M, Sedivy R, Bankl HC, Stiglbauer W, Preusser M, Rossler K, Hainfellner JA (2009) The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol 95(3):401–411
Koperek O, Bergner O, Pichlhofer B, Oberndorfer F, Hainfellner JA, Kaserer K, Horvat R, Harris AL, Niederle B, Birner P (2011) Expression of hypoxia-associated proteins in sporadic medullary thyroid cancer is associated with desmoplastic stroma reaction and lymph node metastasis and may indicate somatic mutations in the VHL gene. J Pathol 225(1):63–72
Preusser M, Heinzl H, Gelpi E, Schonegger K, Haberler C, Birner P, Marosi C, Hegi M, Gorlia T, Hainfellner JA (2006) Histopathologic assessment of hot-spot microvessel density and vascular patterns in glioblastoma: poor observer agreement limits clinical utility as prognostic factors: a translational research project of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Cancer 107(1):162–170
Preusser M, Heinzl H, Gelpi E, Hoftberger R, Fischer I, Pipp I, Milenkovic I, Wohrer A, Popovici F, Wolfsberger S, Hainfellner JA (2008) Ki67 index in intracranial ependymoma: a promising histopathological candidate biomarker. Histopathology 53(1):39–47
Preusser M, Hoeftberger R, Woehrer A, Gelpi E, Kouwenhoven M, Kros JM, Sanson M, Idbaih A, Brandes AA, Heinzl H, Gorlia T, Hainfellner JA, van den Bent M (2012) Prognostic value of Ki67 index in anaplastic oligodendroglial tumours—a translational study of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Histopathology 60(6):885–894
Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I, Ludovini V, Magrini E, Gregorc V, Doglioni C, Sidoni A, Tonato M, Franklin WA, Crino L, Bunn PA Jr, Varella-Garcia M (2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst 97(9):643–655
Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21(20):3798–3807
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29(2):134–141
Soffietti R, Ruda R, Trevisan E (2008) Brain metastases: current management and new developments. Curr Opin Oncol 20(6):676–684
Schoenegger K, Oberndorfer S, Wuschitz B, Struhal W, Hainfellner J, Prayer D, Heinzl H, Lahrmann H, Marosi C, Grisold W (2009) Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol 16(7):874–878
Villa S, Weber DC, Moretones C, Manes A, Combescure C, Jove J, Puyalto P, Cuadras P, Bruna J, Verger E, Balana C, Graus F (2011) Validation of the new Graded Prognostic Assessment scale for brain metastases: a multicenter prospective study. Radiat Oncol 6:23
Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF (2005) MR imaging correlates of survival in patients with high-grade gliomas. Am J Neuroradiol 26(10):2466–2474
Carrillo JA, Lai A, Nghiemphu PL, Kim HJ, Phillips HS, Kharbanda S, Moftakhar P, Lalaezari S, Yong W, Ellingson BM, Cloughesy TF, Pope WB (2012) Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. Am J Neuroradiol 33(7):1349
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2):190–198
Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27(1):65–73
Ryken TC, McDermott M, Robinson PD, Ammirati M, Andrews DW, Asher AL, Burri SH, Cobbs CS, Gaspar LE, Kondziolka D, Linskey ME, Loeffler JS, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Kalkanis SN (2010) The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96(1):103–114
Preusser M, de Ribaupierre S, Wohrer A, Erridge SC, Hegi M, Weller M, Stupp R (2011) Current concepts and management of glioblastoma. Ann Neurol 70(1):9–21
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16(1):116–122
Steeg PS, Camphausen KA, Smith QR (2011) Brain metastases as preventive and therapeutic targets. Nat Rev Cancer 11(5):352–363
Leenders WP, Kusters B, Verrijp K, Maass C, Wesseling P, Heerschap A, Ruiter D, Ryan A, de Waal R (2004) Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res 10(18 Pt 1):6222–6230
Carbonell WS, Ansorge O, Sibson N, Muschel R (2009) The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4(6):e5857
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307
Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Davis DW, McConkey DJ, Fidler IJ (2000) Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res 60(17):4959–4967
Diehn M, Nardini C, Wang DS, McGovern S, Jayaraman M, Liang Y, Aldape K, Cha S, Kuo MD (2008) Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci USA 105(13):5213–5218
Acknowledgments
This study was performed within the framework of the Society of Austrian Neurooncology (SANO, www.sano.co.at). This study was supported by grant number 13457 of the Austrian National Bank (principle investigator: Matthias Preusser).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spanberger, T., Berghoff, A.S., Dinhof, C. et al. Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis 30, 357–368 (2013). https://doi.org/10.1007/s10585-012-9542-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9542-9