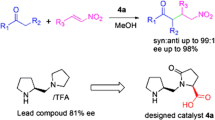
Abstract
Interaction of enamine intermediate of primary or secondary amine with carbonyl compound and formation of hydrogen bonds with electronegative atoms are often responsible to activate a nucleophile or electrophile to enhance enantio- and diastereoselectivity in typical organocatalytic asymmetric transformations. The review lists some suitable applications of enamine intermediate and hydrogen bonding interaction in asymmetric organocatalysis.





































Similar content being viewed by others
References
Ahrendt KA, Borths CJ, MacMillan DWC (2000) New strategies for organic catalysis: the first highly enantioselective organocatalytic Diels–Alder reaction. J Am Chem Soc 122:4243–4244
King HD, Meng Z, Denhart D, Mattson R, Kimura R, Wu D, Gao Q, Macor JE (2005) Enantioselective synthesis of a highly potent selective serotonin reuptake inhibitor. an application of imidazolidinone catalysis to the alkylation of indoles with an α, β-disubstituted α, β-unsaturated aldehyde. Org Lett 7:3437–3440
Jang HY, Hong JB, MacMillan DWC (2007) Enantioselective organocatalytic singly occupied molecular orbital activation: the enantioselective α-enolation of aldehydes. J Am Chem Soc 129:7004–7005
Reisman SE, Doyle AG, Jacobsen EN (2008) Enantioselective thiourea-catalyzed additions to oxocarbenium ions. J Am Chem Soc 130:7198–7199
Simon L, Goodman JM (2011) A model for the enantioselectivity of imine reactions catalyzed by BINOL-phosphoric acid catalysts. J Org Chem 76:1775–1788
Mukherjee S, Yang JW, Hoffmann S, List B (2007) Asymmetric enamine catalysis. Chem Rev 107:5471–5569
Ramachary DB, Reddy YV (2012) Dienamine catalysis: an emerging technology in organic synthesis. Eur J Org Chem 2012:865–887
Jia ZJ, Jiang H, Li JL, Gschwend B, Li QZ, Yin X, Grouleff J, Chen YC, Jørgensen KA (2011) Trienamines in asymmetric organocatalysis: Diels–Alder and tandem reactions. J Am Chem Soc 133:5053–5061
Erkkilä A, Majander I, Pihko PM (2007) Iminium catalysis. Chem Rev 107:5416–5470
Dalko PL, Moisan L (2004) In the golden age of organocatalysis. Angew Chem Int Ed 43:5138–5175
Lee JH, Deng L (2012) Asymmetric approach toward chiral cyclohex-2-enones from anisoles via an enantioselective isomerization by a new chiral diamine catalyst. J Am Chem Soc 134:18209–18212
Liu L, Sarkisian R, Xu ZH, Wang H (2012) Asymmetric Michael addition of ketones to alkylidene malonates and allylidene malonates via enamine–metal Lewis acid bifunctional catalysis. J Org Chem 77:7693–7699
Tsogoeva SB, Wei S (2006) During the final stages of preparation of this manuscript, a different primary amine-thiourea catalyst system was reported for analogous ketonenitroalkene Michael reactions. Chem Commun 1451–1453
Etter MC, Urbanczyk-Lipkowska Z, Zia-Ebrahimi M, Panunto TW (1990) Hydrogen bond-directed cocrystallization and molecular recognition properties of diarylureas. J Am Chem Soc 112:8415–8426
Bow WF, Basak AK, Jolit A, Vicic DA, Tius MA (2010) Enamine-iminium ion Nazarov cyclization of r-ketoenones. Org Lett 12:440–443
Luo SZ, Li JY, Xu H, Zhang L, Cheng JP (2007) Chiral amine-polyoxometalate hybrids as highly efficient and recoverable asymmetric enamine catalysts. Org Lett 9:3675–3678
Bhadury PS, Yao YY, He YG (2012) Organocatalytic application of axially dissymmetric BINOLs and their conversion into binaphthyl phosphoric acids. Curr Org Chem 16:1730–1753
Roy S, Chen K (2012) Three-component organocascade kinetic resolution of racemic nitroallylic acetates via sequential iminium/enamine asymmetric catalysis. Org Lett 14:2496–2499
Momiyama N, Yamamoto Y, Yamamoto H (2007) Diastereo- and enantioselective synthesis of nitroso Diels-Alder-type bicycloketones using dienamine: mechanistic insight into sequential nitroso Aldol/Michael reaction and application for optically pure 1-amino-3,4-diol synthesis. J Am Chem Soc 129:1190–1195
Matsui K, Takizawa S, Sasai H (2005) Bifunctional organocatalysts for enantioselective aza-Morita–Baylis –Hillman reaction. J Am Chem Soc 127:3680–3681
Takizawa S, Horii A, Sasai H (2010) Acid–base organocatalysts for the aza-Morita–Baylis–Hillman reaction of nitroalkenes. Tetrahedron Asymmetry 21:891–894
Yamanaka M, Hirata T (2009) DFT study on bifunctional chiral Brønsted acid-catalyzed asymmetric hydrophosphonylation of imines. J Org Chem 74:3266–3271
Simon L, Goodman JM (2008) Theoretical study of the mechanism of Hantzsch ester hydrogenation of imines catalyzed by chiral BINOL-phosphoric acids. J Am Chem Soc 130:8741–8747
Lin P, Song BA, Bhadury PS, Hu DY, Zhang YP, Jin LH, Yang S (2008) Chiral separation of novel α-aminophosphonates containing a benzothiazole moiety by liquid chromatography using an amylose stationary phase. Chin J Chem 26:1659–1665
Coric I, Kim JH, Vlaar T, Patil M, Thiel W, List B (2013) Brosted acid catalyzed asymmetric sn2-type o-alkylations. Angew Chem Int Ed 52:3490–3493
Shi F, Xing GJ, Tan W, Zhu RY, Tu SJ (2013) Enantioselective construction of 2,5-dihydropyrrole skeleton with quaternary stereogenic center via catalytic asymmetric 1,3-dipolar cycloaddition involving α-arylglycine esters. Org Biomol Chem 11:1482–1489
Chen XH, Zhang WQ, Gong LZ (2008) Asymmetric organocatalytic three-component 1,3-dipolar cycloaddition: control of stereochemistry via a chiral Brønsted acid activated dipole. J Am Chem Soc 130:5652–5653
Momiyama N, Konno T, Furiya Y, Iwamoto T, Terada M (2011) Design of chiral bis-phosphoric acid catalyst derived from (R)-3,3′-di(2-hydroxy-3-arylphenyl) binaphthol: catalytic enantioselective Diels-Alder reaction of α, β-unsaturated aldehydes with amidodienes. J Am Chem Soc 133:19294–19297
Russo A, Lattanzi A (2010) Asymmetric organocatalytic conjugate addition of diarylphosphane oxides to chalcones. Eur J Org Chem 2010:6736–6739
Chauhan P, Chimni SS (2010) Asymmetric addition of indoles to isatins catalysed by bifunctional modified cinchona alkaloid catalysts. Chem Eur J 16:7709–7713
Hiemstra H, Wynberg H (1981) Addition of aromatic thiols to conjugated cycloalkenones, catalyzed by chiral beta-hydroxy amines. A mechanistic study of homogeneous catalytic asymmetric synthesis. J Am Chem Soc 103:417–430
Hintermann L, Ackerstaff J, Boeck F (2013) Inner workings of a cinchona alkaloid catalyzed oxa-Michael cyclization: evidence for a concerted hydrogen-bond-network mechanism. Chem Eur J 19:2311–2321
Zhu Q, Lu YX (2010) Stereocontrolled creation of all-carbon quaternary stereocenters by organocatalytic conjugate addition of oxindoles to vinyl sulfone. Angew Chem Int Ed 49:7753–7756
Huang HB, Jacobsen EN (2006) Highly enantioselective direct conjugate addition of ketones to nitroalkenes promoted by a chiral primary amine-thiourea catalyst. J Am Chem Soc 128:7170–7171
Luo SZ, Xu H, Li JY, Zhang L, Cheng JP (2007) A simple primary-tertiary diamine-Brønsted acid catalyst for asymmetric direct Aldol reactions of linear aliphatic ketones. J Am Chem Soc 129:3074–3075
Melchiorre P (2012) Cinchona-based primary amine catalysis in the asymmetric functionalization of carbonyl compounds. Angew Chem Int Ed 51:9748–9770
Jiang L, Chen Y-C (2011) Recent advances in asymmetric catalysis with cinchona alkaloid -based primary. Catal Sci Technol 1:354–365
Bencivenni G, Galzerano P, Mazzanti A, Bartoli G, Melchiorre P (2010) Direct asymmetric vinylogous Michael addition of cyclic enones to nitroalkenes via dienamine catalysis. Proc Natl Acad Sci USA 107:20642–20647
Bastida D, Liu YK, Tian X, Eduardo EA, Melchiorre P (2013) Asymmetric vinylogous aldol reaction via H-bond-directing dienamine catalysis. Org Lett 15:220–223
Acknowledgments
We are grateful for financial support received from the National Natural Science Foundation of China (No. 21062005); Guizhou University Personnel Research foundation (No. 201122) and Education Ministry Foundation of Guizhou Province [No. KY (2011) 232].
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yao, Y., Chen, M., Zhang, X. et al. Organocatalytic Application of Enamine Intermediate and Hydrogen Bonding Interaction to Dissymmetric Transformation. Catal Surv Asia 18, 34–49 (2014). https://doi.org/10.1007/s10563-013-9162-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-013-9162-7