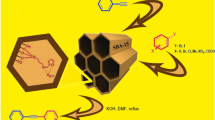
Abstract
We report for the first time a simple, highly efficient and ecofriendly approach for the preparation of pyrrole derivatives in the presence of dendritic amine grafted on mesoporous silica under solvent free condition. We have synthesized and characterized the three generations of dendritic amine grafted on mesoporous silica (G0–G2) through a stepwise growth technique. The maximum yield was observed with third generation (G2) and the catalyst was easily separated from the reaction mixture. Studies showed that dendritic effect of amino groups on the catalyst was the driving force of the reaction pathway, which has led to the formation of pyrrole derivatives. The green aspect of the present method are use of metal free catalyst, low catalyst loading, shorter reaction times, organic solvents are not needed, facile work-up, purification of the products by non-chromatographic methods, the excellent yield of the products, room temperature and the reusability of the catalyst.
Graphical Abstract












Similar content being viewed by others
References
Bhagiyalakshmi M, Park SD, Cha WS, Jang HT (2010) Appl Surf Sci 256:6660
King ASH, Twyman LJ (2002) J Chem Soc 1: 2209
Esfahani MN, Baltork IM, Khosropour AR, Moghadam M, Mirkhani V, Tangestaninejad S (2013) J Mol Catal A 379: 243–254
Aghapoor K, Nia LE, Mohsenzadeh F, Morad MM, Balavar Y, Darabi HR (2012) J Organomet Chem 25: 708
Ferre M, Pleixats R, Chi Man MW, Cattoen X (2016) Green Chem 18: 881
Chermahini AN, Omran MK, Dabbagh HA, Mohammadnezhad G, Teimouri A (2015) New J Chem 39:4814
Lang LM, Li BJ, Liu W, Jiang L, Xu Z, Yin G (2010) Chem Commun 46:448
Algarra M, Jimenez MV, Rodriguez-Castellon E, Jimenez-Lopez A, Jimenez J (2005) Chemosphere 59:779
Mazloum-Ardakani M, Sheikh-Mohseni MA, Abdollahi-Alibeik M, Benvidi A (2012) Analyst 137:1950
Xie T, Shi L, Zhang J, Zhang D (2014) Chem Commun 507250
Sinija P S, Sreekumar K (2015) RSC Adv 5: 101176.
Bhardwaj V, Gumber D, Abbot V, Dhimana S, Sharmaa P 2015 RSC Adv 5:15233.
Kamal A, Faazil S, Malik MS, Balakrishna M, Bajee S, Siddiqui MRH, Alarifi A (2016) Arabian J Chem 9:542.
Nun P, Dupuy S, Gaillard S, Poater A, Cavallod L, Nolan SP (2011) Catal Sci Technol 1:58.
Forberg D, Obenauf J, Friedrich M, Huhne S, Mader W, Motzc G, Kempe R (2014) Catal Sci Technol 4:4188.
Palacios F, Aparico D, Santos JM, Vicario JM (2001) Tetrahedron 57:1961
Estevez V, Villacampa M, Menendez JC (2013) Chem Commun 49: 591
Dieter RK, Yu H (2000) Org Lett 2: 2283
Arcadi A, Rossi E (1998) Tetrahedron 54: 15253
Lee CF, Yang LM, Hwu TY, Feng AS, Tseng JC, Luh TY (2000) J Am Chem Soc 122:4992
Katritzky A, Jiang J, Steel PJ (1994) J Org Chem 59:4551
Paul S, Das AR (2012) Catal Sci Technol 2: 1130
Dong Y, Naranjan N, Ablaza SL, Yu SX, Bolvig S, Forsyth DA, Le Quesne PW (1999) J Org Chem 64: 2657
Haubmann C, Huebner H, Gmeiner P (1999) Bioorg. Med. Chem. Lett. 9: 3143
Robertson J, Hatley RJD, Watkin DJ (2000) J Chem Soc 1: 3389
Wurtz NR, Turner JM, Baird EE, Dervan PB (2001) Org Lett 3: 1201
Chen J, Liu M, Yang X, Ding J, Wu H (2008) J Braz Chem Soc 19: 877
Dou G, Shi C, Shi D (2008) J Comb Chem 10: 810
Bharadwaj AR, Scheidt KA (2004) Org Lett 6: 2465
Minetto G, Raveglia LF, Taddei M (2004) Org Lett 6: 3
Akelis L, Rousseau J, Juskenas R, Dodonova J, Rousseau C, Menuel S, Prevost D, Tumkevicius S, Monflier E, Hapio F (2016) Eur J Org Chem. 1: 31
Duan F, Ding J, Deng H, Chen D, Liu JCM, Wu H (2013) Chin Chem Lett 24: 793
Neelakandeswari N, Sangami G, Emayavaramban P, Karvembu R, Dharmaraj N, Kim HY(2012) Tetrahedron Lett 53:2980
Jalal S, Sarkar S, Bera K, Maiti S, Jana U (2013) Eur J Org Chem 4823.
Phan NTS, Nguyen TT, Luu QH, Nguyen LTL 2012 J. Mol. Catal. A: Chem. 363: pp 178
Darabi HR, Poorheravi MR, Aghapoor K, Mirzaee A, Mohsenzadeh F, Asadollahnejad N, Taherzadeh H, Balavar Y 2012 Environ. Chem. Lett. 10: pp 5
Duan FJ, Ding JC, Deng HJ, Chen DB, Chen JX, Liu MC, Wu HY 2014 Chin Chem Lett 24793
Su P, Chiu S, Lin Y 2016 Sens. Actuators, B224: 833.
Rahmatpour A (2012) J Organomet Chem 712:15
Polshettiwar V, Baruwati B, Varma RS 2009 Chem. Commun.1837
Kim BH, Bae S, Go A, Lee H, Gong C, Lee BM 2016 Org Biomol Chem 14: 265
Cho H, Madden R, Nisanci B, Torok B 2015 Green Chem 17: 1088
Bora U, Saikia A, Boruah RC (2003) Org Lett 5: 435.
Cho H, Torok F, Torok B (2014) Green Chem 16: 3623.
Abid M, Spaeth A, Tcrck (2006) Adv Synth Catal 348:2191
Abid M, Landge SM, Toriik (2006) Org Prep Proced Int 35: 495.
Banik M, Ramirez B, Reddy A, Bandyopadhyay D, Banikl BK (2012) Org Med Chem Lett 2:11.
Eftekhari-Sis B, Akbari A, Amirabed M (2011) Chem Heterocycl Compd 46: 11.
Lapina I M, Pevzner LM, Potekhin AA,(2007) Russ J Gen Chem 77: 5.
Makowska-Janusik M, Kassiba A, Errien N, Mehdi A 2010 J Inorg Organomet Polym. 20: pp 761
Darabi HR, Aghapoor K, Farahani AD, Mohsenzadeh F 2012 Environ Chem Lett 10: 369
Gao L, Bing L, Zhang Z, Kecheng H, Xiaoyun H, Deng K 2013 J. Organomet. Chem. 735: 26
Jafari AA, Mahmoudi H 2013 Environ Chem Lett 11: 157
Handy S, Lavender K (2013) Tetrahedron Lett 54: 4377.
Cheraghi S, Saberi D, Heydari A 2014 Catal Lett 144: 1339
Zhang X, Weng G, Zhang Y, Li Y 2015 Tetrahedron 71: 2595
Samadi M, Behbahani FK (2015) J. Chil. Chem Soc 60.
Sherly PB, Sreekumar K unpublished results
Abbat S, Dhaked D, Arfeen M, Bharatam PV 2015 RSC Adv 5: 88353.
Acknowledgements
We would like to thank STIC, CUSAT, Dept. of Physics, CUSAT and IIT Delhi for assistance with various analyses. One of the authors (K. A. J) thanks the CSIR, Govt. of India for financial assistance in the form of fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jisha, K.A., Sreekumar, K. Dendritic Amine on Mesoporous Silica: First Organo Base Catalyst for Paal Knorr Reaction under Solvent Free Condition, A green approach. Catal Lett 147, 964–975 (2017). https://doi.org/10.1007/s10562-017-1975-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-1975-y