Abstract
In the present study, poly(HEA-co-DVB) was synthesized (loading of OH groups: 8.07 mmol OH/g, crosslinking degree: 6 % DVB) and used for N-hydroxyphthalimide (NHPI) immobilization via ester bond (NHPI loading: 2.06 mmol NHPI/g). The obtained polymeric support and poly(HEA-co-DVB)/NHPI catalyst were characterized by FT-IR and XPS spectroscopy, elemental analysis, thermogravimetry and particle size measurement. The novel polymer-supported NHPI catalyst was tested in aerobic oxidation of p-methoxytoluene and α-methylstyrene. The studied reactions were carried out in the presence of AIBN and/or Co(II) salt without solvent or in acetic acid as solvent. It was found that the obtained polymer-supported NHPI catalyst showed the best catalytic performance, which was attributed to a high content of accessible NHPI moieties. The recovery and recycling of the obtained HEA/DVB-NHPI was only possible in reactions proceeded in solvent-free conditions.
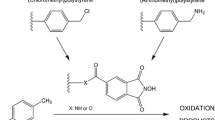
Graphical Abstract

Similar content being viewed by others
1 Introduction
N-hydroxyphthalimide (NHPI) has been known as a valuable catalyst for aerobic oxidation of various organic compounds [1–3]. Its catalytic effect has been demonstrated among others in oxidation of alkylaromatics, such as toluene, ethylbenzene, cumene and their derivatives, to respective alcohols, ketones, carboxylic acids or hydroperoxides. Recently NHPI was also applied as catalyst for oxidative cleavage of α-methylstyrenes [4]. The activity of NHPI is related to formation of phthalimide N-oxyl radical (PINO) that abstract hydrogen from substrate as shown in Scheme 1 [5]. Rate of H-abstraction by PINO is higher than by peroxyl radical in autocatalytic oxidation [e.g. rate constants for H-abstraction from toluene by PINO and ROO· are 0.08 and 0.21 (mol−1 s−1) at 25 °C, respectively] [6]. Additionally the catalytic effect is a result of the lower termination rate because of ROO· radicals’ reaction with NHPI in place and of their recombination to stable products.
Mechanism of hydrocarbon oxidation in the presence of NHPI [5]
PINO formation is usually facilitated by additives such as: transition metals [7, 8], azo-compounds [9–11], peroxides [12, 13], aldehydes [14], enzymes [15, 16] and others [17]. For instance, when the most popular combination, i.e. NHPI and Co(II) salt, was applied in toluene oxidation with oxygen, benzoic acid was obtained in yield of 81 % at 25 °C (0.1 MPa, 20 h) [18]. In industry, benzoic acid is obtained in the presence of Amoco catalyst at significantly higher temperature (190 °C, 1.5 MPa). The role of transition metal salts is initiation of reaction by PINO generation as well as catalyzing the decomposition of formed peroxy compounds into stable products as shown in reactions Eqs. 2 and 6 [18]. Metal-free additives, such as azo-compounds, peroxides and aldehydes, act only as initiators and impact on the PINO generation.
Recently, much attention has been paid to heterogeneous catalysts. Application of both immobilized NHPI and metal co-catalyst or combination of homogeneous NHPI and immobilized transition metal co-catalysts has been reported. These catalytic systems are especially attractive due to simple separation from the reaction mixture. Examples of heterogeneous NHPI catalysts include NHPI immobilized on silica gel by physical interaction [19], NHPI immobilized by chemical bonds on silica gel [20, 21], polystyrene [22], copolymer microspheres of glycidyl and methyl methacrylate (GMA/MMA) [23] or glycidoxypropyl-SBA-15 [24] and NHPI incorporated into metal organic framework [25–29]. On the other hand, silica supported cobalt(II) [30, 31], cobalt and manganese supported hexagonal mesoporous silicas [32] or cobalt Shiff base complex anchored on starch-coated magnetic nanoparticles [33] were also examined in the presence of homogeneous NHPI. The activity of these catalytic systems was demonstrated in aerobic oxidations of cyclohexane and other cyclic hydrocarbons [19, 25, 27–29, 32], toluenes [20–22, 24], ethylbenzenes [23, 25, 30, 31, 33], styrenes [26] as well as other alkyl aromatic compounds [29, 31, 33] and benzylic alcohols [33]. For example, when toluene was oxidized with oxygen in the presence of both NHPI and Co(II) chemically bonded to silica gel in AcOH as solvent, conversion of 18 % was achieved (100 °C, 0.1 MPa, 20 h) [20]. Similar conversion was obtained when toluene was oxidized under pressure of oxygen in the presence of N,N-dihydroxypyromellitimide (NDHPI) supported on glycidoxypropyl-SBA-15 in MeCN (Co(II), 70 °C, 1.6 MPa, 7 h) [24]. When NHPI immobilized on commercial polystyrene via amide or ester bonds was applied in p-methoxytoluene oxidation with oxygen in AcOH or solvent-free condition conversions of 18 and 2.3 %, respectively, were achieved (Co(II), 80 °C, 0.1 MPa, 6 h) [22]. The final effect of NHPI immobilization on a solid carrier by means of covalent bond depends on quantity, quality and distribution of functional groups in a carrier. The cross-linked and thus insoluble synthetic functional polymers are attractive as solid carriers for catalysts and biocatalysts, because they can be formed into grains of predetermined shape and dimensions. The pendant functional groups are used for the formation of stable covalent bonds with molecules of a catalyst. The pendant functional groups in commercially available polystyrenes, such as NH2 and OH, have been previously used by us to immobilize trimellitic anhydride acid chloride (TAC), which is the precursor of NHPI [22]. Nevertheless, commercially available polymers contain only small amount of these functional groups. In the polymeric beads based on cross-linked 2-hydroxyethylacrylate (HEA), containing high density of hydroxyethyl groups can be employed to immobilize of NHPI via ester bonds. 2-Hydroxyethylacrylate (HEA) is the closest analogue of 2-hydroxyethylmethacrylate (HEMA). Vinyl monomers are also commercially available and are commonly used for synthesis of three-dimensional hydrophilic copolymer networks called hydrogels, synthesized via three-dimensional free-radical polymerization (TFRP) [34]. Hydrogels HEMA and HEA-based, due to good mechanical properties, no cytotoxicity, biocompatibility and high thermal stability are applied as biomaterials and coatings [35–39]. In particular, the HEMA is the subject of numerous original papers and monographs on the synthesis, properties and applications of HEMA-based hydrogels, such as the reviewed article of Montheard et al. [40]. The first and the most spectacular application of HEMA-based hydrogels are soft contact lenses developed by Otto Wichterle in 1960 [41–44]. The immobilization of NHPI on the polymer carriers based on HEMA and HEA is not reported in literature, but HEMA-based polymers have been used to immobilize the enzymes [45–47]. Three-dimensional, free-radical copolymerization of HMA and HEA with divinyl monomers called cross-linkers can be carried out using a variety of techniques: bulk cross-linking copolymerization [39, 48], solution cross-linking copolymerization [49, 50] and suspension cross-linking copolymerization [51]. They showed that HEMA-based hydrogel cross-linked of divinylbenzene (DVB) was the smallest deformable polymer tested in this study and had the highest value of Young’s modulus. According to these results, it appears that DVB is the appropriate cross-linker for the synthesis of HEA-based carrier to immobilize NHPI.
In this paper, the application of poly(HEA-co-DVB) as support for NHPI has been reported for the first time. In contrary to previously reported the polystyrene-supported NHPI [22] and GMA/MMA-supported NHPI [23], the new catalyst HEA/DVB-NHPI is characterized by higher NHPI loading. The formed linker is longer than in polystyrene and similar to GMA/MMA supported N–OH groups. There is no data on NHPI loading in other described chemically immobilized NHPI [20, 21, 24]. Herein, activity of this new solid HEA/DVB-NHPI has been studied in oxidation reactions of p-methoxytoluene and α-methylstyrene.
2 Experimental
2.1 Materials
2-Hydroxyethylacrylate (HEA, 96 %, Aldrich), divinylbenzene (technical 55 %, Sigma–Aldrich), benzoyl peroxide (75 %, Sigma–Aldrich), trimellitic anhydride chloride (98 %, Sigma–Aldrich), hydroxylamine hydrochloride (98 %, Sigma–Aldrich) N-Hydroxyphthalimide (97 %, Sigma–Aldrich), 2,2′-azobis(2-methylpropionitrile) (AIBN, 98 %, Acros Organics), Co(OAc)2·H2O (pure p. a., POCH),were used as received. Solvents Dichloromethane (DCM, Pure p. a., POCH), Pyridine (Pure p. a., POCH), N,N-dimethylformamide (DMF, Pure p. a., POCH), tetrahydrofuran (THF, Pure p. a., POCH), diethyl ether (Et2O Pure p. a., POCH), methanol (MeOH Pure p. a., POCH), dichloroethane (DCE Pure p. a., POCH), acetic acid (AcOH, Pure p.a., POCH), acetonitrile (MeCN, pure p. a., POCH). Reagents were used as received without further purification. Solvents used in catalytic tests such as p-methoxytoluene (99.8 %, Acros Organics), α-methylstyrene (98 %, Sigma–Aldrich) were purified and dried with standard methods.
2.2 Preparation of Catalyst
2.2.1 Polymerization of 2-Hydroxyethylacrylate with Divinylbenzene
Copolymer of HEA and DVB (poly(HEA-co-DVB)) was prepared by mixing HEA (3.00 mL, 26 mmol) and DVB (0.40 mL, 1.54 mmol), then BPO was added (0.48 g, 2 mmol). The solution was transferred into a 30 mL vial. The vial was flushed with argon and sealed. The vial was heated at 60 °C for 6 h. The copolymer was removed from vial, crushed, washed with DCM and dried under vacuum.As references [17], [55] and [39], [49] are duplicate, we have deleted the duplicate references and renumbered accordingly. Please check and confirm.Now is ok
FT-IR of HEA/DVB: 3392, 2947, 2877, 1721, 1448, 1393, 1239, 1239, 1159, 1073, 888, 841, 756, 741 cm−1. Elemental analysis of HEA/DVB: C 54.12 %, H 7.13 %, another 38.75 %.
2.2.2 Immobilization of Trimellitic Anhydride Chloride
The mixture of copolymer poly(HEA-co-DVB) (1.00 g), DCM (30 mL) and pyridine (0.83 mL, 10 mmol) was cooled to 0 °C in ice bath. Next, solution of TAC (2.17 g, 10 mmol) in 30 mL DCM was added dropwise over 1 h. The reaction mixture was stirred for 3 h at 0 °C and for additional 24 h at RT. The product was filtered and washed twice with 10 mL of solvent in the following order: DCM, DMF, THF, DCM and Et2O. Product-copolymer poly(HEA-co-DVB) with immobilized TAC (HEA/DVB-TAC) was dried under vacuum. Next, HEA/DVB-TAC was added to a 40 mL mixture of pyridine: DCE (3:1, v/v), followed by the addition of hydroxylamine hydrochloride (0.71 g, 10 mmol). The mixture was stirred for 24 h at 75 °C. The product was filtered and washed with H2O (2 × 10 mL), MeOH (2 × 10 mL), DMF (2 × 10 mL), DMF/water (1:1, v/v, 2 × 10 mL), DMF (1 × 10 mL), THF (3 × 10 mL), DCM (2 × 10 mL), and Et2O (2 × 10 mL) and dried under vacuum. Copolymer poly(HEA-co-DVB) with immobilized NHPI (HEA/DVB-NHPI) was obtained.
FT-IR of HEA/DVB-TAC: 2950, 1852, 1777, 1725, 1456, 1296, 1266, 1228, 1166, 1102, 930, 888, 786, 717, 695 cm−1. Elemental analysis of HEA/DVB-TAC: C 53.81 %, H 4.89 %, another 41.30 %.
FT-IR of HEA/DVB-NHPI: 3218, 2937, 1785, 1725, 1454, 1336, 1292, 1253, 1185, 1159, 1104, 1062, 989, 921, 882, 770, 706 cm−1. Elemental analysis of HEA/DVB-NHPI: C 56.13 %, H 4.91 %, N 2.89 %, another 36.07 %.
Swelling ratio of HEA/DVB-NHPI was determined at 8.4 % in AcOH as a solvent, while there was no swelling of polymer in p-methoxytoluene and α-methylstyrene observed. Particle size was measured and mean size was 143 μm (D [3, 4]), distribution of particles was in the range between 56.7 and 255.8 μm (d(0.1): 56.667 μm; d(0.5): 124.8 μm; d(0.9): 255.8 μm) (Fig. 1). Specific surface area of obtained catalyst was 0.0721 m2/g.
2.3 Typical Procedure for Oxidation Without Solvent
The oxidation reactions were performed in a gasometric apparatus described in Ref. [52]. p-Methoxytoluene (2.0 mL, 15.9 mmol) or α-methylstyrene (2.0 mL, 16.3 mmol), HEA/DVB-NHPI (0.10 g), AIBN (0.03 mmol) and/or Co(OAc)2 ·H2O (0.02 mmol) were placed in a flask connected to a gas burette filled with oxygen under atmospheric pressure. The mixture was heated up to reaction temperature and stirred at 1400 rpm for 6 or 15 h. The oxygen uptake was measured and recalculated to the conditions of 0 °C and 1 atm. After the reaction, the catalyst was separated through filtration, washed with MeCN and DCM and then recycled. After recycling HEA/DVB-NHPI was characterized by FT-IR. The oxidation products were determined by GC analysis.
2.4 Typical Procedure for Oxidation in AcOH
p-Methoxytoluene (0.63 mL, 5 mmol), AcOH (10 mL), HEA/DVB-NHPI (0.10 g), cobalt(II) acetate (Co(OAc)2⋅H2O, 0.02 mmol) and AIBN (0.03 mmol) were placed in a flask connected to a gasometric apparatus. Reaction was conducted at 80 °C for 6 h. The oxidation procedure was the same as for the oxidation described above.
2.5 Analytical Methods
Infrared spectra were recorded on a Nicolet 6700 FT-IR Spectrometer. Elemental analysis was performed on a CHNS Vario Micro Cube. GC analysis of products of p-methoxytoluene oxidation was performed using an Agilent Technologies 7890A/5975C Gas Chromatography (HP-5 MS capillary column, 30 m × 0.25 mm × 0.25 µm, helium 1.2 mL/min) with FID detector. The injection port temperature was 250 °C. The detector temperature was 280 °C. The temperature program was: hold at 50 °C for 5 min, ramp at 5 °C min−1 to 200 °C, then ramp at 25 °C min−1 to 300 °C for 5 min. Conversion and selectivity were calculated based on internal standard-cyclohexanone. GC analysis of products of α-methylstyrene oxidation was performed using a Hewlett 5890 Series II Gas Chromatography (Zebron ZB-5HT capillary column, 30 m × 0.32 mm × 0.1 µm, helium 48 kPa) with FID detector. The injection port temperature was 280 °C. The detector temperature was 280 °C. The temperature program was: hold at 70 °C for 2 min, ramp at 5 °C min−1 to 100 °C, then ramp at 20 °C min−1 to 250 °C for 2 min. Conversion and selectivity were calculated based on internal standard-naphthalene.
Thermogravimetric analysis (TG) was carried out in SDT q600 thermobalance (TA instrument). The measurement was performed in corundum crucibles using about 10 mg of sample. The decomposition of the organic part was monitored in an air flow (100 × mL/min) while the temperature was increased from 30 to 1000 °C at a rate of 20 °C min−1. Particle size and surface area were measured on MASTERSIZER 2000 MALVERN INSTRUMENTS. The X-ray photoelectron spectra (XPS) were acquired using a Prevac photoelectron spectrometer equipped with a hemispherical VG SCIENTA R3000 analyzer. The spectra were taken using a monochromatized aluminum source Al Kα (E = 1486.6 eV) and a low-energy electron flood gun (FS40A-PS) to compensate for the charge accumulation on the surface of nonconductive samples. Spectra were processed and deconvoluted using CasaXPS software.
3 Results and Discussion
In this paper, new polymer—supported NHPI was obtained and examined in selected oxidation reactions. Copolymer poly(HEA-co-DVB) was used to immobilized NHPI via ester bond.
3.1 Preparation of Catalyst
Copolymer poly(HEA-co-DVB) was synthesized by polymerization of HEA and DVB, then NHPI was immobilized on prepared HEA/DVB as shown in Scheme 2. The FT-IR spectrum (Fig. 2I) of HEA/DVB showed characteristic peaks of hydroxyl and carbonyl groups at 3392 and 1721 cm−1, respectively. After immobilization of TAC, the peak of hydroxyl group at 3392 cm−1 disappeared on FT-IR spectrum (Fig. 2II). The FT-IR spectrum of Fig. 2II. showed new peaks at 1852 and 1777 cm−1 corresponding to anhydride groups [53] and 1228 cm−1—stretching vibrations of C–O bond. The appearance of new anhydride bonds as well as disappearance of hydroxyl group bond confirmed immobilization of TAC. FT-IR spectrum of final product—HEA/DVB-NHPI showed peak at 3218 cm−1 (Fig. 2III) corresponding to hydroxyl group in obtained N-hydroxyphthalimide moiety. Moreover, characteristic bond of anhydride group at 1852 cm−1 disappeared and carbonyl bond at 1777 cm−1 (O = C(O)) shifts to 1785 cm−1 (O = C(N)). Peaks at 3218 and 1785 cm−1 confirmed formation of N-hydroxyl group and obtaining immobilized form of N-hydroxyphthalimide [17, 54].
The XPS analysis was performed to confirm the presence of catalytic centers on the surface of material. The presence of N 1 s peak in obtained catalyst confirmed the immobilization of NHPI onto polymeric support. The high-resolution N 1 s spectra collected for NHPI as well as for HEA/DVB-NHPI are displayed on Fig. 3. The N 1 s core level spectra were fitted with only one component. In the case of NHPI binding energy of N–OH is 401.4 eV and in HEA/DVB-NHPI binding energy of N–OH group is 401.5 eV.
Based on elemental analysis, loading of hydroxyethyl groups in HEA/DVB and cross-linking degree were calculated as 8.07 mmol OH/g and 6 % DVB, respectively. The results of elemental analysis were also used to calculate NHPI loading of 2.06 mmol NHPI/g.
Loading of immobilized NHPI was lower than expected based on high amount of –OH loading in synthesized support. However, NHPI loading and cross-linking degree of HEA/DVB-NHPI were higher in comparison to reported NHPI supported on commercially available (chloromethyl)polystyrene via ester bond (maximum loading 1.65 mmol NHPI/g, maximum cross-linking degree 5.5 % DVB) [22]. In contrary to our obtained heterogeneous NHPI, GMA/MMA-NHPI microspheres were characterized by lower amount of NHPI previously bonded through a Schiff base reaction (1.1 mmol/g) and ethylene glycol dimethacrylate as cross-linker (no data of cross-linking degree) [23]. There is no data on NHPI loading in other described chemically immobilized NHPI [20, 21, 24].
Stability of polymer was investigated by Thermogravimetric analysis (Fig. 4). TG spectra can be distinguished by two regions of mass loss: (I) up to 150 °C which corresponding to physically adsorbed water, (II) above 150 °C which corresponding to desorption and oxidation of material. Three stages of weight loss in TG curve over 150 °C were observed: (a) area of 150–250 °C showed start of catalyst decomposition, (b) area between 250 and 500 °C showed major weight loss which were characteristic for carbonization of sample and c) area above 500 °C was characterized as carbon oxidation. TG analysis demonstrated stability of obtained immobilized NHPI in temperature of performed oxidation reactions.
3.2 Catalytic Tests of HEA/DVB-NHPI
p-Methoxytoluene and α-methylstyrene were used as starting material for catalytic tests of obtained new polymer-supported NHPI. Oxidation of model compounds was performed in the presence of HEA/DVB-NHPI both in polar solvent as well as in solvent-free conditions. Reactions were performed at temperature in the range of 70–90 °C in the presence of AIBN or AIBN and Co(II) salt. Oxygen uptakes were used to observe reactions progress and were collected in Tables 1 and 2. Immobilized NHPI was recycled up to three times. Inconsiderable loss of catalyst during filtration was observed due to small mesh size of catalyst and low scale of performed oxidation.
It was proved that obtained HEA/DVB-NHPI could successfully catalyzed oxidation processes of p-methoxytoluene and α-methylstyrene in solvent-free conditions. Studied reactions were performed in the presence of AIBN or AIBN/Co(II) system. AIBN was added in the amount of 0.1–0.2 mol% in order to initiate the oxidation process and reduce the induction period. AIBN decomposed to alkyl radicals (2-cyanoprop-2-yl radicals) under the applied conditions that could abstract hydrogen mainly from NHPI. For instance, when p-methoxytoluene was oxidized in the presence of HEA/DVB-NHPI and Co(II) salt without AIBN, indication period of 30 min was observed at 80 °C. It was reduced to 9 min after AIBN addition. Effect of Co(II) salt in oxidation of both p-methoxytoluene and α-methylstyrene in the presence of HEA/DVB-NHPI was studied. When reaction of p-methoxytoluene with HEA/DVB-NHPI/Co(II) system was performed the higher O2 consumption were achieved (Table 1, entries 9, 16). Additionally, induction period was reduced from 14 to 7 min. In oxidation of α-methylstyrene similar oxygen consumption was obtained in the presence of HEA/DVB-NHP/Co(II) system and without additive of metal salt (Table 2, entries 8, 12). Similarly, no effect of Co(II) salt additive on NHPI-catalyzed oxidation of α-methylstyrene was reported in paper [4].
The higher catalytic effect of HEA/DVB-NHPI was observed in oxidation of p-methoxytoluene than α-methylstyrene. For example, consumption of O2 in p-methoxytoluene at 80 °C increased up to 15.5 times (Table 1, entry17) in comparison to reaction performed without HEA/DVB-NHPI (Table 1, entry 15). As expected, the rate of oxidation reaction of α-methylstyrene was higher than p-methoxytoluene. However, the increased of O2 consumption was lower, for example at 70 °C increased only up to 1.7 times (Table 2, entry 3) in comparison to reaction without NHPI (Table 2, entry 1).
The catalytic activity of immobilized NHPI was similar in subsequent recycles (Table 1, entries 16–19; Table 2, entries 2–4), which could prove stability of novel heterogeneous organocatalyst at the temperatures up to 80 °C. It was observed that catalytic activity of recycled immobilized NHPI was sometimes higher than the fresh one’s. It is assumed that groups presented in solid catalyst that inhibit free radical reaction could be oxidized to inactive once during first usage.
The effect of temperature on oxidation of p-methoxytoluene and α-methylstyrene in the presence of HEA/DVB-NHPI was also examined. As expected, the oxygen uptake increased when temperature rose. However, the O2 consumption in comparison to blank reaction was lower at 90 °C (1.45 times) than at 80 °C (4.25 times) for p-methoxytoluene and at 80 °C (1.1 times) than 70 °C (1.3 times) for α-methylstyrene. It shows that the rate of non-catalytic reactions is higher at the higher temperature.
The effect of amount of immobilized NHPI was studied in oxidation of p-methoxytoluene. HEA/DVB-NHPI at amount of 0.025, 0.05 and 0.15 g caused lower catalytic effect than 0.1 g. An excessive amount of heterogeneous NHPI (0.15 g) in relation to 2 mL of reaction mixture caused worst effectiveness resulted in lower consumption of O2.
Obtained HEA/DVB-NHPI was also used in oxidation of p-methoxytoluene performed in acetic acid as a polar solvent (Table 1, entries 2–4). Previously reported NHPI immobilized on polystyrene by amide or ester bonds demonstrated significantly higher activity in the presence of polar solvent but the activity was lost during recycles [22]. It seemed that higher cross-linked novel HEA/DVB-NHPI would be more stable in solvent conditions. Over 160 times higher oxygen consumption in the process with novel heterogeneous NHPI than in oxidation without NHPI was observed. Unfortunately, catalytic activity of reused HEA/DVB-NHPI significantly decreased. FT-IR analysis of HEA/DVB-NHPI after second recycle in the solvent conditions (Fig. 5II) showed decreasing of the intensity of peaks at 1785, 1785 and 1159 cm−1 assigned to the carbonyl group (O = C(N–OH)) and ester bonds –C–O– (both in immobilized NHPI and in copolymer poly(HEA-co-DVB)). The signal of hydroxyl group at 3392 cm−1, presented in HEA/DVB spectrum (Fig. 1I) beforehand, was observed. Cleavage of the ester bonds in heterogeneous NHPI occurred in contrast to HEA/DVB-NHPI after second recycle in solvent-free system (Fig. 5III). Intensity of both signals of carbonyl groups O = C(O) at 1725 cm−1 and O = C(NOH) at 1785 cm−1 was not changed. Furthermore, peak at 3218 cm−1 characteristic for N-hydroxyl group was strengthened.
The composition of products obtained in oxidation of p-methoxytoluene and α-methylstyrene was determined by GC-FID analysis and presented in Table 3. p-Methoxytoluene was oxidized to p-methoxybenzaldehyde, p-methoxybenzyl alcohol and p-methoxybenzoic acid while main product of α-methylstyrene oxidation was acetophenone.
4 Conclusion
This paper shows that immobilization of NHPI on copolymer poly(HEA-co-DVB) via ester bond has been successfully achieved. The structure of novel heterogeneous catalyst was confirmed by FT-IR and XPS spectroscopy and elemental analysis. Based on the results of elemental analysis the amount of immobilized NHPI and the crosslinking degree of carrier were calculated respectively 2.06 mmol NHPI/g and 6 mol% of DVB. The amount of NHPI immobilized on investigated carrier is higher than on commercially available polystyrenes (MPS-NHPI: 0.35–1.65 mmol NHPI/g) [22] as well as on copolymer of glycidyl methacrylate and methyl methacrylate (GMA/MMA-NHPI: 1.1 mmol NHPI/g) [23]. There is no data about NHPI loading of silica-supported NHPI [20, 21] and NDHPI immobilized on SBA-15 [24]. Additionally, the ester bond between active N-hydroxyl group and investigated carrier has got the ethyl-linker, which is longer than in polystyrene-based carrier [22].
HEA/DVB-NHPI was applied in oxidation p-methoxytoluene in AcOH as solvent. O2 consumption in comparison to NHPI loading of HEA/DVB-NHPI was 16 mmol O2/mmol N–OH (Co(II), 80 °C, 1 atm, 5 h). It was significantly higher than in comparison to previously reported silica-supported NHPI and Co(II) salt used in oxidation of toluene O2, when O2 uptake per NHPI loading was calculated as only 4.8 mmol O2/mmol NOH (80 °C, 1 atm, 20 h) [20]. Unfortunately, HEA/DVB-NHPI was unstable in AcOH due to degradation of the ester bonds in acidic media.
Activity of HEA/DVB-NHPI was also demonstrated in oxidation reactions of p-methoxytoluene and α-methylstyrene in solvent-free system. When reaction was carried out without solvent recovery and recycling of immobilized NHPI were possible. In comparison to previously studies of chemically bonded NHPI [20–24], the obtained HEA/DVB-NHPI represented higher activity per mole of immobilized NHPI. Determined amount of O2 consumed per amount of immobilized NHPI was 3.3 mmol O2/mmol N–OH and 2.7 mmol O2/mmol N–OH in p-methoxytoluene oxidation in the presence of novel HEA/DVB-NHPI (2.06 mmol NHPI/g) and in previously described (chloromethyl)polystyrene-supported NHPI (1.65 mmol NHPI/g), respectively, under the same reaction conditions. However, as results of higher loading of NHPI and longer linker, higher oxygen uptake was achieved when HEA/DVB-NHPI was used.
References
Recupero F, Punta C (2007) Chem Rev 107:3800
Coseri S (2009) Cat Rev Sci Eng 51:218
Chen K, Zhang P, Wang Y, Li H (2014) Green Chem 16:2344
Lin R, Chen F, Jiao N (2012) Org Lett 14:4158
Amorati R, Lucarini M, Mugnaini V, Pedulli GF, Minisci F, Recupero F, Fontana F, Astolfi P, Greci L (2003) J Org Chem 68:1747
Sheldon RA, Arends IWCE (2006) J Mol Catal A: Chem 251:200
Aoki Y, Hirai N, Sakaguchi S, Ishii Y (2005) Tetrahedron 61:10995
Coseri S (2009) Catal Rev 51:218
Aoki Y, Sakaguchi S, Ishii Y (2004) Adv Synth Cata 346:199
Arends IWCE, Sasidharan M, Kühnle A, Duda M, Jost C, Sheldon RA (2002) Tetrahedron 58:9055
Punta C, Rector CL, Porter NA (2005) Chem Res Toxicol 18:349
Tsujimoto S, Iwahama T, Sakaguchi S, Ishii Y (2001) Chem Commun (Camb) 22:2352
Tsujimoto S, Sakaguchi S, Ishii Y (2003) Tetrahedron Lett 44:5601
Minisci F, Gambarotti C, Pierini M, Porta O, Punta C, Recupero F, Lucarini M, Mugnaini V (2006) Tetrahedron Lett 47:1421
d’Acunzo F, Baiocco P, Galli C (2003) New J Chem 27:329
Sakaguchi S, Eikawa M, Ishii Y (1997) Tetrahedron Lett 38:7075
Yang G, Ma Y, Xu J (2004) J Am Chem Soc 126:10542
Yoshino Y, Hayashi Y, Iwahama T, Sakaguchi S, Ishii Y (1997) J Org Chem 62:6810
Hermans I, Van Deun J, Houthoofd K, Peeters J, Jacobs P A (2007) J Catal 251:204
Rajabi F, Clark JH, Karimi B, Macquarrie DJ (2005) Org Biomol Chem 3:725
Ishii Y, Takano M, Hirai N (2010) US Patent 0317869
Kasperczyk K, Orlinska B, Witek E, Latka P, Zawadiak J, Proniewicz L (2015) Catal Lett 145:1856
Gao B, Meng S, Yang X (2015) Org Process Res Dev 19:1374
Zhou M, Li X, Bao L, Yuan X, Luo H (2015) Catal Lett 2:383
Dhakshinamoorthy A, Alvaro M, Garcia H (2011) Chem Eur J 17:6256
Dhakshinamoorthy A, Alvaro M, Garcia H (2011) ACS Catal 1:836
Dhakshinamoorthy A, Alvaro M, Garcia H (2012) J Catal 289:259
Mikami Y, Dhakshinamoorthy A, Alvaro M, Garcia H (2013) Chem Cat Chem 5:1964
Yua P, Liub G, Tangb R (2014) Curr Organocatal 1:79
Rajabi F, Luque R, Clark JH, Karimi B, Macquarrie DJ (2011) Catal Commun 12:510
Chen L, Li B-D, Xu Q-X, Liu D-B (2013) Chin Chem Lett 24:849
Yua WH, Zhoub CH, Tongb DS, Xua TN (2012) J Mol Catal A Chem 365:194
Jafarpour M, Rezaeifard A, Yasinzadeh V, Kargar H (2015) RSC Adv. doi:10.1039/C5RA04718H
Matsumoto A (1995) Springer, Berlin Heidelberg pp 41–80
Monthearda J-P, Chatzopoulosa M, Daniel Ch (1992) J Macromol Sci Pol R 32:1
Horàk D, Jayakrishnan A, Arshady R (2003) In: Arshady R (ed) Polymers in medicine and biology, vol 1. Citus Books, London, pp 80–107
Horàk D (2006) In: Pethrick CA, Zaokov EE (eds) Handbook of polymer research, vol 19. Nova Science Publishers Inc, New York, pp 1–33
Moghdam MN, Pioletti DP (2015) J Biomed Mater Res Part B 00B:000
Meherchi L, Chabane Sari SM, Senoudi AR, Zargou S, Benmouna F (2015) J Mater Environ Sci 6:2221
Montheard JP, Chatzopoulos M, Chappard D (1992) J Macromol Sci. Macromol Rev 32:1
Wichterle O, Lim D (1960) Nature 185:117
Kunzler J, McGee J (1995) Chem Ind 16:651
Wichterle O (1972) US Patent 3,679,504
Kopeček J (2009) J Polym Sci A Polym Chem 47:5929
Basri M, Samsudin S, Bin Ahmad M, Razak CNA, Salleh AB (1999) Appl Biochem Biotech 81:205
Ayhan F, Ayhan H, Piskin E, Tanyolac A (2002) Bioresource Technol 81:131
Hamdy S, El-Sigeny S, Abou TM (2008) J Macromol Sci A 45:980
Serrano AA, Campillo Fernàndeza AJ, Gómez Ribellesa JL, Monleón PM, Gallego FG, Pissisb P (2004) Polymer 45:8949
Monleón PM, Gómez Ribelles JL, Serrano AA, Gallego FG, Suay AJ, Pissis P (2001) Polymer 42:4667
Santander-Borrego M, Green DW, Chirila TV, Andrew K, Whittaker AK, Blakey I (2014) J Polym Sci Pol Chem 52:1781
Okay O, Gurun C (1992) J Appl Polym Sci 46:401
Kasperczyk K, Orlinska B, Zawadiak J (2014) Cent Eur J Chem 12:1176
Konieczynska MD, Dai C, Stephenson CRJ (2012) Org Biomol Chem 10:4509
Krishnakumar V, Manohar S, Nagalakshmi R (2008) Spectrochim Acta A 71:110
Acknowledgments
Financial assistance from the National Science Centre of Poland (Grant No. N N209 755440) is gratefully acknowledged. Part of the research was done with equipment purchased in the frame of European Regional Development Fund (Polish Innovation Economy Operational Program—contract no. POIG.02.01.00-12-023/08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Łątka, P., Kasperczyk, K., Orlińska, B. et al. N-Hydroxyphthalimide Immobilized on Poly(HEA-co-DVB) as Catalyst for Aerobic Oxidation Processes. Catal Lett 146, 1991–2000 (2016). https://doi.org/10.1007/s10562-016-1830-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1830-6