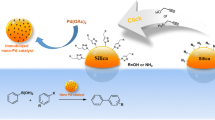
Abstract
Silica-immobilized benzamide palladium complex was prepared and characterized. The complex exhibits excellent catalytic activity and stability for Suzuki–Miyaura cross-coupling reaction under mild aqueous reaction condition. Various aryl bromides were coupled with arylboronic acids in i-PrOH/H2O, under open air at room temperature, in the presence of 0.22 mol% of the catalyst to afford corresponding cross-coupled products in high yields. Furthermore, the novel heterogeneous catalyst can be conveniently recovered by simple filtration and reused several times without significant loss in activity.
Graphical Abstract









Similar content being viewed by others
References
Miyaura N, Suzuki A (1995) Chem Rev 95:2457
Suzuki A (2011) Angew Chem Int Ed 50:6722
Fihri A, Bouhrara M, Nekoueishahraki B, Basset JM, Polshettiwar V (2011) Chem Soc Rev 40:5181
Sellars JD, Steel PG (2011) Chem Soc Rev 40:5170
Maluenda I, Navarro O (2015) Molecules 20:7528
Liu C, Li X (2016) Chem Rec 16:84
Das P, Linert W (2016) Coord Chem Rev 311:1
Nicolaou KC, Bulger PG, Sarlah D (2005) Angew Chem Int Ed 44:4442
Kotha S, Lahiri K, Kashinath D (2002) Tetrahedron 58:9633
Magano J, Dunetz JR (2011) Chem Rev 111:2177
Torborg C, Beller M (2009) Adv Synth Catal 351:3027
Naso F, Babudri F, Farinola GM (1999) Pure Appl Chem 71:1485
Fleckenstein CA, Plenio H (2010) Chem Soc Rev 39:694
Bellina F, Carpita A, Rossi R (2004) Synthesis 15:2419
Kostas ID, Tenchiu AC, Arbez-Dindre C, Psycharis V, Rapropoulou CP (2014) Catal Commun 51:15
Herrmann WA, Reisinger CP, Spiegler MJ (1998) J Organomet Chem 557:93
Marion N, Nolan SP (2008) Acc Chem Res 41:1440
Fortman GC, Nolan SP (2011) Chem Soc Rev 40:5151
Schaper LA, Hock SJ, Herrmann WA, Kühn FE (2013) Angew Chem Int Ed 52:270
Grasa GA, Viciu MS, Huang J, Zhang C, Trudell ML, Nolan SP (2002) Organometallics 21:2866
Zhang J, Zhao L, Song M, Mak TCW, Wu Y (2006) J Organomet Chem 691:1301
Alonso DA, Najera C (2010) Chem Soc Rev 39:2891
Lee S (2006) J Organomet Chem 691:1347
Amadio E, Scrivanti A, Beghetto V, Bertoldini M, Alam MM, Matteoli U (2013) RSC Adv 3:21636
Ye C, Chen Z, Wang H, Wu J (2012) Tetrahedron 68:5197
Tao B, Boykin DW (2004) J Org Chem 69:4330
Li JH, Liu WJ (2004) Org Lett 6:2809
Banik B, Tairai A, Shahnaz N, Das P (2012) Tetrahedron Lett 53:5627
Dewan A, Bora U, Borah G (2014) Tetrahedron Lett 55:1689
Rao GK, Kumar A, Kumar B, Kumar D, Singh AK (2012) Dalton Trans 41:1931
Mondal M, Bora U (2014) Tetrahedron Lett 55:3038
Arumugam V, Kaminsky W, Bhuvanesh NSP, Nallasamy D (2015) RSC Adv 5:59428
Leadbeater NE (2005) Chem Commun 23:2881
Saha D, Chattopadhyay K, Ranu BC (2009) Tetrahedron Lett 50:1003
Mondal M, Bora U (2012) Green Chem 14:1873
Decorrignies A, Fihri A, Azemar G, Djedaini-Pilard F, Len C (2013) Catal Commun 32:101
Xiang L, Xiaohua Z, Ming L (2013) Appl Organometal Chem 27:615
Liu C, Rao X, Song X, Qiu J, Jin Z (2013) RSC Adv 2:526
Liu L, Dong Y, Pang B, Ma J (2014) J Org Chem 79:7193
Sarmah G, Mondal M, Bora U (2015) Appl Organometal Chem 29:495
Paul S, Islam MM, Islam SM (2015) RSC Adv 5:42193
Crudden CM, Sateesh M, Lewis R (2005) J Am Chem Soc 127:10045
Yin L, Liebscher J (2007) Chem Rev 107:133
Faria VW, Oliveira DGM, Kurz MHS, Gonçalves FF, Scheeren CW, Rosa GR (2014) RSC Adv 4:13446
Karami K, Ghasemi M, Naeini NH (2013) Catal Commun 38:10
Nandi M, Uyama H (2014) RSC Adv 4:20847
Le X, Dong Z, Jin Z, Wang Q, Ma J (2014) Catal Commun 53:47
Singh AK, Rao GK, Kumar A, Singh MP, Saleem F, Kumar S (2015) RSC Adv 5:20081
Patel KN, Bedekar AV (2015) Catal Lett 145:1710
Veerakumar P, Velayudham M, Lu KL, Rajagopal S (2013) Appl Catal A 455:247
Martínez A, Krinsky JL, Peñafiel I, Castillón S, Loponov K, Lapkin A, Godard C, Claver C (2015) Catal Sci Technol 5:310
Polshettiwar V, Len C, Fihri A (2009) Coord Chem Rev 253:2599
Sarmah C, Sahu D, Das P (2012) Catal Today 198:197
Begum T, Mondal M, Gogoi PK, Bora U (2015) RSC Adv 2015(5):38085
Dhara K, Sarkar K, Srimani D, Saha SK, Chattopadhyay P, Bhaumik A (2010) Dalton Trans 39:6395
Demel J, Sujandi, Park SE, Cejka J, Stepnicka P (2009) J Mol Catal: A Chem 302:28
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Pure Appl Chem 57:603
Pavia C, Giacalone F, Bivona LA, Salvo AMP, Petrucci C, Strappaveccia G, Vaccaro L, Aprile C, Gruttadauria M (2014) J Mol Cat A: Chem 387:57
Richardson JM, Jones CW (2007) J Catal 251:80
Widegren JA, Finke RG (2003) J Mol Cat A: Chem 198:317
Liu F, Feng G, Lin M, Wang C, Hu B, Qi C (2014) J Colloid Interface Sci 435:83
Whitesides GM, Hackett M, Brainard RL, Lavalleye JPPM, Sowinski AF, Izumi AN, Moore SS, Brown DW, Staudt EM (1985) Organometallics 4:819
Paal C, Hartmann W (1918) Chem Ber 51:711
Anton DR, Crabtree RH (1983) Organometallics 2:855
Lewis LN, Lewis N (1986) J Am Chem Soc 108:7228
Lin Y, Finke RG (1994) Inorg Chem 33:4891
Weddle KS, Aiken JD III, Finke RG (1998) J Am Chem Soc 120:5653
van Asselt R, Elsevier CJ (1991) J Mol Catal 65:L13
Foley P, DiCosimo R, Whitesides GM (1980) J Am Chem Soc 102:6713
Süss-Fink G, Faure M, Ward TR (2002) Angew Chem Int Ed 41:99
Lewis LN (1986) J Am Chem Soc 108:743
Hajipour AR, Shirdashtzade Z, Azizi G (2014) J Chem Sci 126:85
Tong X, Zhao Y, Huang T, Liu H, Liew KY (2009) Appl Surf Sci 255:9463
Kitamura Y, Sako S, Tsutsui A, Monguchi Y, Maegawa T, Kitade Y, Sajiki H (2010) Adv Synth Catal 352:718
Zhou WJ, Wang KH, Wang JX, Gao Z-R (2010) Tetrahedron 66:7633
Acknowledgments
Dr. A. Dewan acknowledges UGC-New Delhi for Dr. D. S. Kothari Postdoctoral fellowship. Dr. M. Mondal and T. Begum are thankful to UGC, New Delhi for financial assistance in the form of UGC-BSR (RFSMS) Fellowship. SAIF (STIC), Cochin is acknowledged for ICP-AES analytical service.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mondal, M., Dewan, A., Begum, T. et al. Suzuki–Miyaura Cross-Coupling in Aqueous Medium Using Recyclable Palladium/Amide-Silica Catalyst. Catal Lett 146, 1718–1728 (2016). https://doi.org/10.1007/s10562-016-1796-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1796-4