Abstract
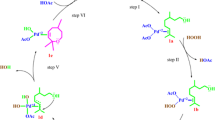
Currently, the oxidative transformations of natural olefins are complicated for competitive reactions such as isomerization, skeletal rearrangements and nucleophilic addition. These concurrent reactions are promoted by metal reoxidant, normally a Lewis acid, and by protic solvent. In this article, the oxidation of terpenes, namely camphene, β-pinene, α-pinene and 3-carene were performed in PdCl2/H2O2/CH3CN system. This metal reoxidant-free system showed highly efficient, especially on camphene and β-pinene oxidation, which resulted in the selective conversion of these substrates into epoxy-derivates and allylic oxidation products, respectively. Additionally, the effects of the principal reactional parameters such as the peroxide/catalyst and substrate/catalyst molar ratio were also investigated.







Similar content being viewed by others
References
Chapuis C, Jacoby D (2001) Appl Catal A 221:93
Gallezot Pierre (2007) Catal Today 121:76
Liu S, Xiao J (2007) J Mol Catal A 270:1
Conley BL, Tenn WJIII, Young KJH, Ganesh SK, Meier SK, Ziatdinov VR, Mironov O, Oxgaard J, Gonzales J, Goddard WAIII, Periana RA (2006) J Mol Catal A 251:8
Cornell CN, Sigman MS (2007) Inorg Chem 46:1903
Sigman MS, Schultz MJ (2004) Org Biomol Chem 2:2551
Hamed O, Henry PM, Thompson C (1999) J Org Chem 64:7745
Schultz MJ, Park CC, Sigman MS (2002) Chem Commun 3034
Mitsudome T, Umetani T, Mori K, Mizugaki T, Ebitani K, Kaneda K (2006) Tetrahedron Lett 47:1425
Gonçalves JA, Bueno AC, Gusevskaya EV (2006) J Mol Catal A 252:5
Muzart J (2006) Chem Asian J 1:508
Nishimura T, Uemura S (2000) Catal Surv Jpn 4:135
Stahl SS (2004) Angew Chem Int Ed 43:3400
Zhan B-Z, Thompson A (2004) Tetrahedron 60:2917
Cornell CN, Sigman MS (2006) Org Lett 8:4117
ten Brink G-J, Arends IWCE, Hoogenraad M, Verspui G, Sheldon RA (2003) Adv Synth Catal 345:1341
Muzart J (2003) Tetrahedron 59:5789
Arends IWCE, Sheldon RA (2002) Topics in Catal 19:133
Escola JM, Botas JA, Aguado J, Serrano DP, Vargas C, Bravo M (2008) Appl Catal A 335:137
Gomes MFT, Antunes OAC (1997) J Mol Catal A 121:145
Uchida T, Matsubara Y, Nishiguchi I, Hirashima T, Ohnishi T, Kanehira K (1990) J Org Chem 55:2939
Caovilla M, Caovilla A, Pergher SBC, Esmelindro MC, Fernandes C, Dariva C, Bernardo-Gusma K, Oestreicher EG, Antunes OAC (2008) Catal Today 133–135:695
Speziali MG, Robles-Dutenhefner PA, Gusevskaya EV (2007) Organomet 26:4003
Gusevskaya EV, Robles-Dutenhefner PA, Ferreira VMS (1998) Appl Catal A 174:177
Maksimcuk NV, Melgunov NS, Mrowiec-Bialon J, Jarzebski AB, Kholdeeva OA (2005) J Catal 235:175
Estrada AC, Simões MMQ, Santos ICMS, Neves MGPMS, Silva MAS, Cavaleiro JAS, Cavaleiro EAMV (2008) Catal Lett (in press)
Bordoloi A, Lefebvre F, Halligudi SB (2007) J Mol Catal A 270:177
Remias JE, Sen A (2002) J Mol Catal A 189:33
Kozitsyna NY, Vargaftik MN, Moiseev II (2000) J. Organomet Chem 593:274
Kuznetsova NI, Kuznetsova LI, Kirillova NV, Detusheva LG, Likholobov VA, Khramov ML, Ansel J-E (2005) Kinetic and Catal 46:204
da Silva MJ, Gonçalves JA, Alves RB, Howarth OW, Gusevskaya EV (2004) J Organomet Chem 689:302
Muzart J (2007) J Mol Catal A 276:62
da Silva MJ, Gonçalves JA, Howarth OW, Gusevskaya EV, Pilo-Veloso D (2005) J Organomet Chem 690:2996
Allal BA, Firdoussi LE, Allaoud S, Karim A, Castanet Y, Mortreux A (2003) J Mol Catal A 200:177
Acknowledgments
Grateful for the financial support from CNPq, FAPEMIG and FUNARBE (Brazil). The authors wish to thank Prof. Sergio Antonio Fernandes and the MS student Lucas Micqueias Arantes for their help in NMR analysis, in addition to Prof. Luis Claudio Barbosa for GC–MS analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, A.A., da Silva, M.L. & da Silva, M.J. Palladium-Catalysed Oxidation of Bicycle Monoterpenes by Hydrogen Peroxide in Acetonitrile Solutions: A Metal Reoxidant-Free and Environmentally Benign Oxidative Process. Catal Lett 130, 424–431 (2009). https://doi.org/10.1007/s10562-009-9970-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-9970-6