Abstract
Purpose
Pathological cardiac remodeling, characterized by cardiac hypertrophy and fibrosis, is a pathological feature of many cardiac disorders that leads to heart failure and cardiac arrest. Vinpocetine, a derivative of the alkaloid vincamine, has been used for enhancing cerebral blood flow to treat cognitive impairment. However, its role in pathological cardiac remodeling remains unknown. The aim of this study is to examine the effect of vinpocetine on pathological cardiac remodeling induced by chronic stimulation with angiotensin II (Ang II).
Methods
Mice received Ang II infusion via osmotic pumps in the presence of vehicle or vinpocetine. Cardiac hypertrophy and fibrosis were assessed by morphological, histological, and biochemical analyses. Mechanistic studies were carried out in vitro with isolated mouse adult cardiac myocytes and fibroblasts.
Results
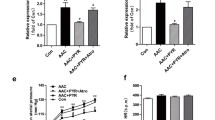
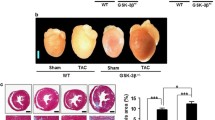
We showed that chronic Ang II infusion caused cardiac hypertrophy and fibrosis, which were all significantly attenuated by systemic administration of vinpocetine. In isolated adult mouse cardiomyocytes, vinpocetine suppressed Ang II-stimulated myocyte hypertrophic growth. In cultured cardiac fibroblasts, vinpocetine suppressed TGFβ-induced fibroblast activation and matrix gene expression, consistent with its effect in attenuating cardiac fibrosis. The effects of vinpocetine on cardiac myocyte hypertrophy and fibroblast activation are likely mediated by targeting cyclic nucleotide phosphodiesterase 1 (PDE1).
Conclusions
Our results reveal a novel protective effect of vinpocetine in attenuating pathological cardiac remodeling through suppressing cardiac myocyte hypertrophic growth and fibroblast activation and fibrotic gene expression. These studies may also shed light on developing novel therapeutic agents for antagonizing pathological cardiac remodeling.






Similar content being viewed by others
References
Leri A, Liu Y, Li B, Fiordaliso F, Malhotra A, Latini R, et al. Up-regulation of AT(1) and AT(2) receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell death. Am J Pathol. 2000;156(5):1663–72.
Klein L, O’Connor CM, Gattis WA, Zampino M, de Luca L, Vitarelli A, et al. Pharmacologic therapy for patients with chronic heart failure and reduced systolic function: review of trials and practical considerations. Am J Cardiol. 2003;91(9A):18F–40F.
Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117(3):568–75.
Bonoczk P, Gulyas B, Adam-Vizi V, Nemes A, Karpati E, Kiss B, et al. Role of sodium channel inhibition in neuroprotection: effect of vinpocetine. Brain Res Bull. 2000;53(3):245–54.
Szobor A, Klein M. Ethyl apovincaminate therapy in neurovascular diseases. Arzneimittelforschung. 1976;26(10a):1984–9.
Patyar S, Prakash A, Modi M, Medhi B. Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep. 2011;63(3):618–28.
Zhang L, Yang L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: a review of the literature. Molecules. 2015;20(1):335–47.
Jeon K-I, Xu X, Aizawa T, Lim JH, Jono H, Kwon D-S, et al. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A. 2010;107(21):9795–800.
Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104(19):2338–43.
Cai Y, Knight WE, Guo S, Li JD, Knight PA, Yan C. Vinpocetine suppresses pathological vascular remodeling by inhibiting vascular smooth muscle cell proliferation and migration. J Pharmacol Exp Ther. 2012;343(2):479–88.
Cai Y, Li JD, Yan C. Vinpocetine attenuates lipid accumulation and atherosclerosis formation. Biochem Biophys Res Commun. 2013;434(3):439–43.
Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105(10):956–64.
Miller CL, Cai Y, Oikawa M, Thomas T, Dostmann WR, Zaccolo M, et al. Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol. 2011;106(6):1023–39.
Wu MP, Zhang YS, Zhou QM, Xiong J, Dong YR, Yan C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of beta2-AR/PI3K/AKT signaling pathway. Pharmacol Res. 2016;104:115–23.
Kabaeva Z, Zhao M, Michele DE. Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. Am J Physiol Heart Circ Physiol. 2008;294(4):H1667–1674.
Yan C, Zhao AZ, Bentley JK, Beavo JA. The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J Biol Chem. 1996;271(41):25699–706.
Knight WE, Chen S, Zhnag Y, Oikawa M, Wu M, Zhou Q, Miller CL, Cai Y, Mickelsen DM, Moravec C, Small EM, Abe J, Yan C. PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc Natl Acad Sci U S A. 2016;pii: 201607728:E7116-7125.
Balestreri R, Fontana L, Astengo F. A double-blind placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular senile cerebral dysfunction. J Am Geriatr Soc. 1987;35(5):425–30.
Crawford DC, Chobanian AV, Brecher P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circ Res. 1994;74(4):727–39.
Kim S, Ohta K, Hamaguchi A, Yukimura T, Miura K, Iwao H. Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension. 1995;25(6):1252–9.
Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, et al. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31(6):1324–30.
Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci U S A. 2000;97(2):931–6.
Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131(11):1019–30.
Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A. Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci U S A. 2001;98(12):6668–73.
Li Y, Ha T, Gao X, Kelley J, Williams DL, Browder IW, et al. NF-kappaB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol. 2004;287(4):H1712–1720.
Onai Y, Suzuki J, Maejima Y, Haraguchi G, Muto S, Itai A, et al. Inhibition of NF-{kappa}B improves left ventricular remodeling and cardiac dysfunction after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292(1):H530–538.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by grants from National Natural Science Foundation of China No.81473379 and No.81673915 (to M. Wu), No. 81570248 (to C. Y.), Shanghai Municipal Bureau of Health Science and Technology Project No. 2011L032B (to M. Wu), the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL111291; HL088400 (to C.Y.) and DC005843, DC004562, and DC013833 (to J.D. Li)].
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethical Approval
All procedures performed in studies involving animals were in accordance with ethical standards of the institution or practice at which the studies were conducted.
Informed Consent
No human subjects are involved.
Additional information
Mei-ping Wu and Yi-shuai Zhang contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 605 kb)
Rights and permissions
About this article
Cite this article
Wu, Mp., Zhang, Ys., Xu, X. et al. Vinpocetine Attenuates Pathological Cardiac Remodeling by Inhibiting Cardiac Hypertrophy and Fibrosis. Cardiovasc Drugs Ther 31, 157–166 (2017). https://doi.org/10.1007/s10557-017-6719-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-017-6719-0