Abstract
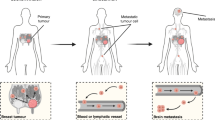
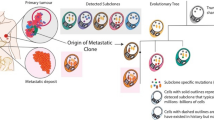
Metastasis is one of the most characteristic yet problematic behaviors of cancer cells. Stage IV breast cancer accounts for a large portion of breast cancer-related morbidity and mortality. Despite early detection and improvement in survival owing to advancements in biomedical research and overall improvement of the health system, 6–10% of patients present with stage IV disease in the developed world, with a higher incidence noted elsewhere. Despite advances in biomedical research into cancer, up to 70–80% of patients with stage IV breast cancer die of cancer in 5 years, a disproportionally higher mortality compared with non-metastatic breast cancer. In this article, we review the incidence, survival, heterogeneity, current practice, and challenges in stage IV breast cancer, and we finish by noting new research initiatives to improve poor survival and suggesting future directions. By doing so, we hope to set the basis of future directions for both treating physicians and translational researchers to relieve the suffering of patients with stage IV breast cancer and improve the survival of patients with this dismal disease.


Similar content being viewed by others
References
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. doi:10.1016/j.cell.2011.02.013.
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nature Reviews Cancer, 3(6), 453–458. doi:10.1038/nrc1098.
Walters, S., Maringe, C., Butler, J., Rachet, B., Barrett-Lee, P., Bergh, J., et al. (2013). Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: a population-based study. British Journal of Cancer, 108(5), 1195–1208. doi:10.1038/bjc.2013.6.
Shulman, L. N., Willett, W., Sievers, A., & Knaul, F. M. (2010). Breast cancer in developing countries: opportunities for improved survival. Journal of Oncology, 2010, 595167. doi:10.1155/2010/595167.
Youlden, D. R., Cramb, S. M., Yip, C. H., & Baade, P. D. (2014). Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biology Medicine, 11(2), 101–115. doi:10.7497/j.issn.2095-3941.2014.02.005.
Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics, 2016. CA: a Cancer Journal for Clinicians, 66(1), 7–30. doi:10.3322/caac.21332.
Di Meglio, A., Freedman, R. A., Lin, N. U., Barry, W. T., Metzger-Filho, O., Keating, N. L., et al. (2016). Time trends in incidence rates and survival of newly diagnosed stage IV breast cancer by tumor histology: a population-based analysis. Breast Cancer Research and Treatment, 157(3), 587–596. doi:10.1007/s10549-016-3845-5.
UK, C. R. (2016). Survival statistics for breast cancer. http://www.cancerresearchuk.org/about-cancer/type/breast-cancer/treatment/statistics-and-outlook-for-breast-cancer-general.
Adisa, A. O., Arowolo, O. A., Akinkuolie, A. A., Titiloye, N. A., Alatise, O. I., Lawal, O. O., et al. (2011). Metastatic breast cancer in a Nigerian tertiary hospital. African Health Sciences, 11(2), 279–284.
Iwasaki, H., & Suda, T. (2009). Cancer stem cells and their niche. Cancer Science, 100(7), 1166–1172 doi:CAS1177.
ECOG-Acrin (2015). E2108: Early Surgery or Standard Palliative Therapy in Treating Patients With Stage IV Breast Cancer. http://ecog-acrin.org/clinical-trials/e2108-educational-materials.
Legha, S. S., Buzdar, A. U., Smith, T. L., Hortobagyi, G. N., Swenerton, K. D., Blumenschein, G. R., et al. (1979). Complete remissions in metastatic breast cancer treated with combination drug therapy. Annals of Internal Medicine, 91(6), 847–852.
Nistico, C., Cuppone, F., Bria, E., Fornier, M., Giannarelli, D., Mottolese, M., et al. (2006). Ten years of experience with weekly chemotherapy in metastatic breast cancer patients: multivariate analysis of prognostic factors. Anti-Cancer Drugs, 17(10), 1193–1200. doi:10.1097/01.cad.0000231485.17063.d3.
Chang, F. (2003). Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (review). International Journal of Oncology, 22(3), 469–480.
Stanford, J. L., Szklo, M., & Brinton, L. A. (1986). Estrogen receptors and breast cancer. Epidemiologic Reviews, 8, 42–59.
Koboldt, D. C., Fulton, R., McLellan, M. D., Schmidt, H., Kalicki-Veizer, J., McMichael, J. F., Fulton, L. L., et al. (2012). Comprehensive molecular portraits of human breast tumours. Nature, 490(7418), 61–70. doi:10.1038/nature11412.
Kimbung, S., Kovacs, A., Danielsson, A., Bendahl, P. O., Lovgren, K., Frostvik Stolt, M., et al. (2015). Contrasting breast cancer molecular subtypes across serial tumor progression stages: biological and prognostic implications. Oncotarget, 6(32), 33306–33318. doi:10.18632/oncotarget.5089.
Engstrom, M. J., Opdahl, S., Hagen, A. I., Romundstad, P. R., Akslen, L. A., Haugen, O. A., et al. (2013). Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Research and Treatment, 140(3), 463–473. doi:10.1007/s10549-013-2647-2.
Kennecke, H., Yerushalmi, R., Woods, R., Cheang, M. C., Voduc, D., Speers, C. H., et al. (2010). Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology, 28(20), 3271–3277. doi:10.1200/jco.2009.25.9820.
De Vita, F., Giuliani, F., Silvestris, N., Catalano, G., Ciardiello, F., & Orditura, M. (2010). Human epidermal growth factor receptor 2 (HER2) in gastric cancer: a new therapeutic target. Cancer Treatment Reviews, 36(Suppl 3), S11–S15. doi:10.1016/S0305-7372(10)70014-1.
Oldenhuis, C. N., Oosting, S. F., Gietema, J. A., & de Vries, E. G. (2008). Prognostic versus predictive value of biomarkers in oncology. European Journal of Cancer, 44(7), 946–953. doi:10.1016/j.ejca.2008.03.006.
van’t Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature, 415(6871), 530–536. doi:10.1038/415530a.
Calemma, R., Ottaiano, A., & Trotta, A. M. E. A. (2012). Fc gamma receptor IIIa polymorphisms in advanced colorectal cancer patients correlated with response to anti-EGFR antibodies and clinical outcome. Journal of Translational Medicine, 10, 232. doi:10.1186/1479-5876-10-232.
Deng, Y., Lin, Y., & Wu, X. (2002). TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes & Development, 16(1), 33–45. doi:10.1101/gad.949602.
Fossati, R., Confalonieri, C., Torri, V., Ghislandi, E., Penna, A., Pistotti, V., et al. (1998). Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. Journal of Clinical Oncology, 16(10), 3439–3460.
Kaufmann, M., Jonat, W., Kleeberg, U., Eiermann, W., Jänicke, F., Hilfrich, J., et al. (1989). Goserelin, a depot gonadotrophin-releasing hormone agonist in the treatment of premenopausal patients with metastatic breast cancer. German Zoladex trial group. Journal of Clinical Oncology, 7(8), 1113–1119.
Grosse-Wilde, A., Voloshanenko, O., Bailey, S. L., Longton, G. M., Schaefer, U., Csernok, A. I., et al. (2008). TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. The Journal of Clinical Investigation, 118(1), 100–110. doi:10.1172/jci33061.
Finnberg, N., Klein-Szanto, A. J., & El-Deiry, W. S. (2008). TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. The Journal of Clinical Investigation, 118(1), 111–123. doi:10.1172/jci29900.
MacFarlane, M., Inoue, S., Kohlhaas, S. L., Majid, A., Harper, N., Kennedy, D. B. J., et al. (2005). Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death and Differentiation, 12(7), 773–782. doi:10.1038/sj.cdd.4401649.
MacFarlane, M., Ahmad, M., Srinivasula, S., Fernandes-Alnemri, T., Cohen, G., Alnemri, E., et al. (1997). Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. The Journal of Biological Chemistry, 272, 25417–25420. doi:10.1074/jbc.272.41.25417.
Mandrekar, S. J., & Sargent, D. J. (2010). Predictive biomarker validation in practice: lessons from real trials. doi:10.1177/1740774510368574.
Manzo, F., Miceli, M., Conte, M., De Bellis, F., Carafa, V., Franci, G., et al. (2009). TNF-related apoptosis-inducing ligand. Signalling of a ‘Smart’ Molecule, 41(3), 460–466. doi:10.1016/j.biocel.2007.12.012.
Jost, P. J., Grabow, S., Gray, D., McKenzie, M. D., Nachbur, U., Huang, D. C. S., et al. (2009). XIAP discriminates between type I and type II FAS-induced apoptosis. Nature, 460(7258), 1035–1039. doi:10.1038/nature08229.
Jin, Z., Dicker, D. T., & El-Deiry, W. S. (2002). Enhanced sensitivity of G1 arrested human cancer cells suggests a novel therapeutic strategy using a combination of simvastatin and TRAIL. Cell Cycle, 1(1), 82–89.
Keating, J., Tsoli, M., Hallahan, A., Ingram, W., Haber, M., Ziegler, D., et al. (2012). Targeting the inhibitor of apoptosis proteins as a novel therapeutic strategy in Medulloblastoma. Molecular Cancer Therapeutics, 11(10), 1–10. doi:10.1158/1535-7163.MCT-12-0352.
Keane, M. M., Rubinstein, Y., Cuello, M., Ettenberg, S. A., Banerjee, P., Nau, M. M., et al. (2000). Inhibition of NF-kappaB activity enhances TRAIL mediated apoptosis in breast cancer cell lines. Breast Cancer Research and Treatment, 64(2), 211–219.
Karacay, B., Sanlioglu, S., Griffith, T. S., Griffith, T. S., Griffith, T. S., Griffith, T. S., et al. (2004). Inhibition of the NF-kappaB pathway enhances TRAIL-mediated apoptosis in neuroblastoma cells. Cancer Gene Therapy, 11(10), 681–690. doi:10.1038/sj.cgt.7700749.
Blair, J. M., Zhou, H., Seibel, M. J., & Dunstan, C. R. (2006). Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nature Reviews Clinical Oncology, 3(1), 41–49. doi:10.1038/ncponc0381.
Kelly, M., Hoel, B., & Voelkel-Johnson, C. (2002). Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer Biology & Therapy, 1(5), 520–527 doi:05240288.
Kerr, J., Winterford, C., & Harmon, B. (1994). Apoptosis. Its significance in cancer and cancer therapy. Cancer, 73(8), 2013–2026. doi:10.1002/1097-0142(19940415)73:8<2013::AID-CNCR2820730802>3.0.CO;2-J.
Kajiwara, K., Saito, A., Ogata, S., & Tanihara, M. (2004). Synthetic peptides corresponding to ligand-binding region of death receptors, DR5, Fas, and TNFR, specifically inhibit cell death mediated by the death ligands, respectively. Biochimica et Biophysica Acta, 1699(1–2), 131–137. doi:10.1016/j.bbapap.2004.02.016.
Plastaras, J. P., Dorsey, J. F., Carroll, K., Kim, S. H., Birnbaum, M. J., & El-Deiry, W. S. (2008). Role of PI3K/Akt signaling in TRAIL- and radiation-induced gastrointestinal apoptosis. Cancer Biology & Therapy, 7(12), 2047–2053.
Pitti, R. M., Marsters, S. A., Ruppert, S., Ruppert, S., Ruppert, S., Ruppert, S., et al. (1996). Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. The Journal of Biological Chemistry, 271(22), 12687–12690.
Phipps, A. I., Buchanan, D. D., Makar, K. W., Burnett-Hartman, A. N., Coghill, A. E., Passarelli, M. N., et al. (2012). BRAF mutation status and survival after colorectal cancer diagnosis according to. Cancer Epidemiology, Biomarkers & Prevention, 21(10), 1792–1798. doi:10.1158/1055-9965.epi-12-0674.
Ravi, R., Bedi, G. C., Engstrom, L. W., Ravi, R., Bedi, G. C., Engstrom, L. W., et al. (2001). Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nature Cell Biology, 3(4), 409–416. doi:10.1038/35070096.
Prasad, S., Yadav, V. R., Kannappan, R., Kannappan, R., Kannappan, R., Kannappan, R., et al. (2011). Ursolic acid, a Pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors. Journ of Biological Chemistry, 286, 5546–5557. doi:10.1074/jbc.M110.183699.
Ricci, S., Jin, Z., Dews, M., Yu, D., Thomas-Tikhonenko, A., Dicker, D., et al. (2004). Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. doi:10.1128/MCB.24.19.8541-8555.2004.
Rimawi, M., Mayer, I., Forero, A., Nanda, R., Goetz, M., Rodriguez, A., et al. (2013). Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2–overexpressing breast cancer: TBCRC 006. doi:10.1200/JCO.2012.44.8027.
Robidoux, A., Tang, G., Rastogi, P., Geyer Jr., C. E., Azar, C. A., Atkins, J. N., et al. (2013). Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. The Lancet Oncology, 14(12), 1183–1192. doi:10.1016/s1470-2045(13)70411-x.
Lee, J., Kim, S., Shin, I., Cho, K., Joo, H., Yoon, C., et al. (2004). Randomized phase III trial of cisplatin, epirubicin, leucovorin, 5-fluorouracil. Cancer Research and Treatment, 36(2), 140–145. doi:10.4143/crt.2004.36.2.140.
Assersohn, L., Salter, J., Powles, T. J., A’Hern, R., Makris, A., Gregory, R. K., et al. (2003). Studies of the potential utility of Ki67 as a predictive molecular marker of clinical response in primary breast cancer. Breast Cancer Research and Treatment, 82(2), 113–123. doi:10.1023/B:BREA.0000003968.45511.3f.
Crump, M., Sawka, C. A., DeBoer, G., Buchanan, R. B., Ingle, J. N., Forbes, J., et al. (1997). An individual patient-based meta-analysis of tamoxifen versus ovarian ablation as first line endocrine therapy for premenopausal women with metastatic breast cancer. Breast Cancer Research and Treatment, 44(3), 201–210.
Yan, S., Li, K., Jiao, X., & Zou, H. (2015). Tamoxifen with ovarian function suppression versus tamoxifen alone as an adjuvant treatment for premenopausal breast cancer: a meta-analysis of published randomized controlled trials. Onco Targets and Therapy, 8, 1433–1441. doi:10.2147/ott.s86817.
Mauri, D., Pavlidis, N., Polyzos, N. P., & Ioannidis, J. P. (2006). Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. Journal of the National Cancer Institute, 98(18), 1285–1291. doi:10.1093/jnci/djj357.
Ellis, M. J., Llombart-Cussac, A., Feltl, D., Dewar, J. A., Jasiowka, M., Hewson, N., et al. (2015). Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. Journal of Clinical Oncology, 33(32), 3781–3787. doi:10.1200/jco.2015.61.5831.
Dauvois, S., White, R., & Parker, M. G. (1993). The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. Journal of Cell Science, 106(Pt 4), 1377–1388.
Robertson, J. F., Llombart-Cussac, A., Rolski, J., Feltl, D., Dewar, J., Macpherson, E., et al. (2009). Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. Journal of Clinical Oncology, 27(27), 4530–4535. doi:10.1200/jco.2008.21.1136.
Mehta, R. S., Barlow, W. E., Albain, K. S., Vandenberg, T. A., Dakhil, S. R., Tirumali, N. R., et al. (2012). Combination anastrozole and fulvestrant in metastatic breast cancer. The New England Journal of Medicine, 367(5), 435–444. doi:10.1056/NEJMoa1201622.
Pang, B., Cheng, S., Sun, S. P., An, C., Liu, Z. Y., Feng, X., et al. (2014). Prognostic role of PIK3CA mutations and their association with hormone receptor expression in breast cancer: a meta-analysis. Scientific Reports, 4, 6255. doi:10.1038/srep06255.
Sanchez, C. G., Ma, C. X., Crowder, R. J., Guintoli, T., Phommaly, C., Gao, F., et al. (2011). Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Research, 13(2), R21. doi:10.1186/bcr2833.
Baselga, J., Semiglazov, V., van Dam, P., Manikhas, A., Bellet, M., Mayordomo, J., et al. (2009). Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. Journal of Clinical Oncology, 27(16), 2630–2637. doi:10.1200/jco.2008.18.8391.
Finn, R. S., Crown, J. P., Lang, I., Boer, K., Bondarenko, I. M., Kulyk, S. O., et al. (2015). The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. The Lancet Oncology, 16(1), 25–35. doi:10.1016/s1470-2045(14)71159-3.
Finn, R.S., Martin, M., Rugo, H.S., Jones, S.E., Im, S-A., Gelmon, K.A., et al. (2016). PALOMA-2: Primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2– advanced breast cancer (ABC). J Clin Oncol, suppl; abstr 507.
Dickler, M., Tolaney, S., Rugo, H., Cortes, J., Dieras, V., Patt, D., et al. (2016). MONARCH1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy. ASCO annual meeting.
Yardley, D. A., Ismail-Khan, R. R., Melichar, B., Lichinitser, M., Munster, P. N., Klein, P. M., et al. (2013). Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. Journal of Clinical Oncology, 31(17), 2128–2135. doi:10.1200/jco.2012.43.7251.
Kornblith, A. B., Hollis, D. R., Zuckerman, E., Lyss, A. P., Canellos, G. P., Cooper, M. R., et al. (1993). Effect of megestrol acetate on quality of life in a dose-response trial in women with advanced breast cancer. The cancer and leukemia group B. Journal of Clinical Oncology, 11(11), 2081–2089.
Cochrane, D. R., Bernales, S., Jacobsen, B. M., Cittelly, D. M., Howe, E. N., D’Amato, N. C., et al. (2014). Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. [research article]. Breast Cancer Research, 16(1).
Andre, F., Broglio, K., Pusztai, L., Berrada, N., Mackey, J. R., Nabholtz, J. M., et al. (2010). Estrogen receptor expression and docetaxel efficacy in patients with metastatic breast cancer: a pooled analysis of four randomized trials. The Oncologist, 15(5), 476–483. doi:10.1634/theoncologist.2009-0150.
Kamal, A. H., Camacho, F., Anderson, R., Wei, W., Balkrishnan, R., & Kimmick, G. (2012). Similar survival with single-agent capecitabine or taxane in first-line therapy for metastatic breast cancer. Breast Cancer Research and Treatment, 134(1), 371–378. doi:10.1007/s10549-012-2037-1.
Liu, M., Mo, Q. G., Wei, C. Y., Qin, Q. H., Huang, Z., & He, J. (2013). Platinum-based chemotherapy in triple-negative breast cancer: a meta-analysis. Oncology Letters, 5(3), 983–991. doi:10.3892/ol.2012.1093.
Chandarlapaty, S., Chen, D., He, W., Sung, P., Samoila, A., You, D., et al. (2016). Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncology. doi:10.1001/jamaoncol.2016.1279.
Andersson, M., Lidbrink, E., Bjerre, K., et al. (2011). Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2–positive breast cancer: the HERNATA study. Journal of Clinical Oncology. doi:10.1200/JCO.2010.30.8213.
Lu, Y., Zi, X., Zhao, Y., Mascarenhas, D., & Pollak, M. (2001). Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). Journal of the National Cancer Institute, 93(24), 1852–1857.
Ritter, C. A., Bianco, R., Dugger, T., Forbes, J., Qu, S., Rinehart, C., et al. (2004). Mechanisms of resistance development against trastuzumab (Herceptin) in an in vivo breast cancer model. International Journal of Clinical Pharmacology and Therapeutics, 42(11), 642–643.
Shattuck, D. L., Miller, J. K., Carraway 3rd, K. L., & Sweeney, C. (2008). Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Research, 68(5), 1471–1477 doi:68/5/1471.
Rexer, B. N., & Arteaga, C. L. (2012). Intrinsic and acquired resistance to HER2-targeted therapies in HER2 Gene-amplified breast cancer: mechanisms and clinical implications. Critical Reviews in Oncogenesis, 17(1), 1–16.
Scaltriti, M., Rojo, F., Ocaña, A., Anido, J., Guzman, M., Cortes, J., et al. (2007). Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. JNCI, 99(8). doi:10.1093/jnci/djk134.
Verma, S., Miles, D., Gianni, L., Krop, I. E., Welslau, M., Baselga, J., et al. (2012). Trastuzumab emtansine for HER2-positive advanced breast cancer. The New England Journal of Medicine, 367(19), 1783–1791. doi:10.1056/NEJMoa1209124.
Scheuer, W., Friess, T., Burtscher, H., Bossenmaier, B., Endl, J., & Hasmann, M. (2009). Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Research, 69(24), 9330–9336. doi:10.1158/0008-5472.CAN-08-4597.
Guan, Z., Xu, B., DeSilvio, M. L., Shen, Z., Arpornwirat, W., Tong, Z., et al. (2013). Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Journal of Clinical Oncology, 31(16), 1947–1953. doi:10.1200/jco.2011.40.5241.
Blackwell, K. L., Burstein, H. J., Storniolo, A. M., Rugo, H. S., Sledge, G., Aktan, G., et al. (2012). Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 study. Journal of Clinical Oncology, 30(21), 2585–2592. doi:10.1200/jco.2011.35.6725.
Blackwell, K. L., Burstein, H. J., Storniolo, A. M., Rugo, H., Sledge, G., Koehler, M., et al. (2010). Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. Journal of Clinical Oncology, 28(7), 1124–1130. doi:10.1200/jco.2008.21.4437.
Krop, I. E., Kim, S. B., Gonzalez-Martin, A., LoRusso, P. M., Ferrero, J. M., Smitt, M., et al. (2014). Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. The Lancet Oncology, 15(7), 689–699. doi:10.1016/s1470-2045(14)70178-0.
Sparano, J. A., Makhson, A. N., Semiglazov, V. F., Tjulandin, S. A., Balashova, O. I., Bondarenko, I. N., et al. (2009). Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: results from a randomized phase III study. Journal of Clinical Oncology, 27(27), 4522–4529. doi:10.1200/jco.2008.20.5013.
Mauri, D., Kamposioras, K., Tsali, L., Bristianou, M., Valachis, A., Karathanasi, I., et al. (2010). Overall survival benefit for weekly vs. three-weekly taxanes regimens in advanced breast cancer: a meta-analysis. Cancer Treatment Reviews, 36(1), 69–74. doi:10.1016/j.ctrv.2009.10.006.
O’Shaughnessy, J., Miles, D., Vukelja, S., Moiseyenko, V., Ayoub, J. P., Cervantes, G., et al. (2002). Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. Journal of Clinical Oncology, 20(12), 2812–2823.
Valero, V., Vrdoljak, E., Xu, B., Thomas, E., Gomez, H., Manikhas, A., et al. (2012). Maintenance of clinical efficacy after dose reduction of ixabepilone plus capecitabine in patients with anthracycline- and taxane-resistant metastatic breast cancer: a retrospective analysis of pooled data from 2 phase III randomized clinical trials. Clinical Breast Cancer, 12(4), 240–246. doi:10.1016/j.clbc.2012.03.013.
Andreopoulou, E., & Sparano, J. A. (2013). Chemotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer: an overview. Current Breast Cancer Reports, 5(1), 42–50. doi:10.1007/s12609-012-0097-1.
de Ruijter, T., Veeck, J., de Hoon, J., van Engeland, M., & Tjan-Heijnen, V. (2011). Characteristics of triple-negative breast cancer. Journal of Cancer Research and Clinical Oncology, 137(2), 183–192. doi:10.1007/s00432-010-0957-x.
Kassam, F., Enright, K., Dent, R., Dranitsaris, G., Myers, J., Flynn, C., et al. (2009). Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clinical Breast Cancer, 9(1), 29–33. doi:10.3816/CBC.2009.n.005.
Dent, R., Trudeau, M., Pritchard, K. I., Hanna, W. M., Kahn, H. K., Sawka, C. A., et al. (2007). Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical Cancer Research, 13(15 Pt 1), 4429–4434. doi:10.1158/1078-0432.ccr-06-3045.
D’Andrea, A. D., & Grompe, M. (2003). The Fanconi anaemia/BRCA pathway. Nature Reviews. Cancer, 3(1), 23–34. doi:10.1038/nrc970.
Staudacher, L., Cottu, P. H., Dieras, V., Vincent-Salomon, A., Guilhaume, M. N., Escalup, L., et al. (2011). Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Annals of Oncology, 22(4), 848–856. doi:10.1093/annonc/mdq461.
Feng, F. Y., de Bono, J. S., Rubin, M. A., & Knudsen, K. E. (2015). Chromatin to clinic: the molecular rationale for PARP1 inhibitor function. Molecular Cell, 58(6), 925–934. doi:10.1016/j.molcel.2015.04.016.
Search of: PARP TNBC trials-ClinicalTrials.gov (2016). https://clinicaltrials.gov/ct2/results?term=PARP+TNBC&Search=Search.
Loi, S., Sirtaine, N., Piette, F., Salgado, R., Viale, G., Van Eenoo, F., et al. (2013). Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. Journal of Clinical Oncology, 31(7), 860–867. doi:10.1200/jco.2011.41.0902.
Search of: TNBC immunotherapy trials - ClinicalTrials.gov (2016). https://clinicaltrials.gov/ct2/results?term=TNBC+immune&Search=Search.
Lehmann, B. D., Bauer, J. A., Chen, X., Sanders, M. E., Chakravarthy, A. B., Shyr, Y., et al. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of Clinical Investigation, 121(7), 2750–2767. doi:10.1172/JCI45014.
Masuda, H., Baggerly, K. A., Wang, Y., Zhang, Y., Gonzalez-Angulo, A. M., Meric-Bernstam, F., et al. (2013). Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clinical Cancer Research, 19(19), 5533–5540. doi:10.1158/1078-0432.CCR-13-0799.
Network, T. C. G. A. (2012). Comprehensive molecular portraits of human breast tumours. Nature, 490, 61–70. doi:10.1038/nature11412.
Stone, P., Richardson, A., Ream, E., Smith, A. G., Kerr, D. J., & Kearney, N. (2000). Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Annals of Oncology, 11(8), 971–975.
Park, Y. H., Jung, K. H., Im, S. A., Sohn, J. H., Ro, J., Ahn, J. H., et al. (2013). Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. Journal of Clinical Oncology, 31(14), 1732–1739. doi:10.1200/jco.2012.45.2490.
Meric-Bernstam, F., Brusco, L., Shaw, K., Horombe, C., Kopetz, S., Davies, M. A., et al. (2015). Feasibility of large-scale genomic testing to facilitate enrollment onto Genomically matched clinical trials. Journal of Clinical Oncology, 33(25), 2753–2762. doi:10.1200/jco.2014.60.4165.
Kris, M. G., Johnson, B. E., Berry, L. D., Kwiatkowski, D. J., Iafrate, A. J., Wistuba, I. I., et al. (2014). Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA, 311(19), 1998–2006. doi:10.1001/jama.2014.3741.
Andre, F., Bachelot, T., Commo, F., Campone, M., Arnedos, M., Dieras, V., et al. (2014). Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). The Lancet Oncology, 15(3), 267–274. doi:10.1016/S1470-2045(13)70611-9.
Garrison, H. H., & Deschamps, A. M. (2014). NIH research funding and early career physician scientists: continuing challenges in the twenty-first century. The FASEB Journal, 28(3), 1049–1058. doi:10.1096/fj.13-241687.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, B., Hortobagyi, G.N. Current challenges of metastatic breast cancer. Cancer Metastasis Rev 35, 495–514 (2016). https://doi.org/10.1007/s10555-016-9636-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9636-y