Abstract
Purpose
Reflex human papillomavirus (HPV) testing is the preferred triage option for most women diagnosed with atypical squamous cells of undetermined significance (ASC-US). This study was conducted to describe follow-up results of women with ASC-US Pap test results in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP), focusing on HPV test use.
Methods
We examined the follow-up of 45,049 women in the NBCCEDP with ASC-US Pap tests during 2009–2011. Data on demographic characteristics, diagnostic procedures, and clinical outcomes were analyzed.
Results
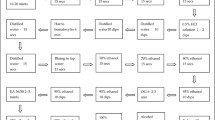
NBCCEDP providers diagnosed 45,049 women (4.5 % of all Pap tests) with an ASC-US result. Of those, 28,271 (62.8 %) were followed with an HPV test, 3,883 (8.6 %) with a repeat Pap test, 6,592 (14.6 %) with colposcopy, and 6,303 were lost to follow-up (14.0 %). Women aged 40 and older were followed more often with an HPV test. White, black, and Asian/Pacific Islander women were followed more often with an HPV test after an ASC-US Pap compared to Hispanic and American Indian/Alaska Native (AI/AN) women. Among women with a positive HPV test on follow-up, almost 90 % continued with colposcopy as recommended. AI/AN women had the highest rates of HPV positivity (55.2 %) and of no follow-up (25.0 %).
Conclusion
This is the first analysis describing follow-up of ASC-US Pap test results in the NBCCEDP, providing a window into current management of ASC-US results. Findings raise concerns about persistent disparities among AI/AN women. During 2009–2011, nearly two-thirds of women with an ASC-US Pap test result were followed with an HPV reflex test.


Similar content being viewed by others
References
Solomon D, Schiffman M, Tarone R, group AS (2001) Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 93(4):293–299
American Cancer Society (2014) Finding cervical pre-cancers. http://www.cancer.org/cancer/cervicalcancer/moreinformation/cervicalcancerpreventionandearlydetection/cervical-cancer-prevention-and-early-detection-find-pre-cancer-changes. Accessed 12 Mar 2014
Gatscha RM, Abadi M, Babore S, Chhieng D, Miller MJ, Saigo PE (2001) Smears diagnosed as ASCUS: interobserver variation and follow-up. Diagn Cytopathol 25(2):138–140
Raab SS, Bishop NS, Zaleski MS (1999) Long-term outcome and relative risk in women with atypical squamous cells of undetermined significance. Am J Clin Pathol 112(1):57–62
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D, American Society for C, Cervical Pathology-sponsored Consensus C (2007) 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol 197(4):346–355. doi:10.1016/j.ajog.2007.07.047
Mullen J, Lantz P (2014) Overview of the National Breast and Cervical Cancer Early Detection Program. Cancer Causes Control. doi:10.1007/s10552-015-0565-9
Gutman SI (2003) Premarket Approval of Digene Hybrid Capture 2 High-Risk HPV DNA Test. http://www.accessdata.fda.gov/cdrh_docs/pdf/p890064s009a.pdf
Ryerson AB, Benard VB, Major AC (2014) Summarizing the first 12 years of partnerships and progress against breast and cervical cancer 1991–2002 national report, vol 24 Mar 2014
Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS Jr, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER, Committee A-A-ACCG (2012) American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 62(3):147–172. doi:10.3322/caac.21139
Research Triangle Institute (2012) SUDAAN language manual, volumes 1 and 2, release 11. Research Triangle Institute, Research Triangle Park
Graubard BI, Korn EL (1999) Predictive margins with survey data. Biometrics 55(2):652–659
Roland KB, Benard VB, Greek A, Hawkins NA, Manninen D, Saraiya M (2013) Primary care provider practices and beliefs related to cervical cancer screening with the HPV test in federally qualified health centers. Prev Med 57(5):419–425. doi:10.1016/j.ypmed.2013.04.012
Apgar BS, Kittendorf AL, Bettcher CM, Wong J, Kaufman AJ (2009) Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. Am Fam Physician 80(2):147–155
Benard VB, Howe W, Saraiya M, Helsel W, Lawson HW (2008) Assessment of follow-up for low-grade cytological abnormalities in the National Breast and Cervical Cancer Early Detection Program, 2000–2005. J Low Genit Tract Dis 12(4):300–306. doi:10.1097/LGT.0b013e31817e308e
Benard VB, Saraiya MS, Soman A, Roland KB, Yabroff KR, Miller J (2011) Cancer screening practices among physicians in the national breast and cervical cancer early detection program. J Womens Health (Larchmt) 20(10):1479–1484. doi:10.1089/jwh.2010.2530
Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, Solomon D, Wentzensen N, Lawson HW, Conference ACG (2013) 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 17(5 Suppl 1):S1–S27. doi:10.1097/LGT.0b013e318287d329
Group A-LTS (2003) A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol 188(6):1393–1400
Watson M, Benard VB, Thomas C, Brayboy A, Paisano R, Becker T (2014) Cervical cancer incidence and mortality among American Indian and Alaska Native Women, 1999–2009. Am J Public Health 104:S415
Alfonsi GA, Datta SD, Mickiewicz T, Koutsky LA, Ghanem K, Hagensee M, Kerndt P, Hsu K, Weinstock H, Shlay JC (2011) Prevalence of high-risk HPV types and abnormal cervical cytology in American Indian/Alaska Native women, 2003–2005. Public Health Rep 126(3):330–337
Benard VB, Watson M, Castle PE, Saraiya M (2012) Cervical carcinoma rates among young females in the United States. Obstet Gynecol 120(5):1117–1123. doi:10.1097/AOG.0b013e31826e4609
Group T, Sharp L, Cotton S, Cochran C, Gray N, Little J, Neal K, Cruickshank M (2009) After-effects reported by women following colposcopy, cervical biopsies and LLETZ: results from the TOMBOLA trial. BJOG 116(11):1506–1514. doi:10.1111/j.1471-0528.2009.02263.x
Reilly R, Paranjothy S, Beer H, Brooks CJ, Fielder HM, Lyons RA (2012) Birth outcomes following treatment for precancerous changes to the cervix: a population-based record linkage study. BJOG 119(2):236–244. doi:10.1111/j.1471-0528.2011.03052.x
Trivers KF, Benard VB, Eheman CR, Royalty JE, Ekwueme DU, Lawson HW (2009) Repeat pap testing and colposcopic biopsies in the underserved. Obstet Gynecol 114(5):1049–1056. doi:10.1097/AOG.0b013e3181b8fc88
Acknowledgments
We would like to thank William Helsel and William Kammerer from Information Management Services, Inc. for their assistance with data analysis. This research was supported in part by an appointment (LL) to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Rights and permissions
About this article
Cite this article
Watson, M., Benard, V., Lin, L. et al. Provider management of equivocal cervical cancer screening results among underserved women, 2009–2011: follow-up of atypical squamous cells of undetermined significance. Cancer Causes Control 26, 759–764 (2015). https://doi.org/10.1007/s10552-015-0549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0549-9