Abstract
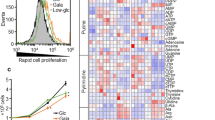
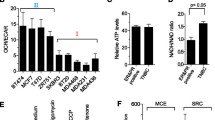
We hypothesized that normal mitochondria inhibited cancer cell proliferation and increased drug sensitivity by the mechanism of suppression of cancer aerobic glycolysis. To demonstrate the mechanism, we used real-time PCR and glycolysis cell-based assay to measure gene expression of glycolytic enzymes and glucose transporters, and extracellular lactate production of human breast cancer cells. We found that isolated fluorescent probe-stained mitochondria of MCF-12A (human mammary epithelia) could enter into human breast cancer cell lines MCF-7, T47D, and MDA-MB-231, confirmed by fluorescent and confocal microscopy. Mitochondria from the untransformed human mammary epithelia increased drug sensitivity of MCF-7 cells to paclitaxel. Real-time PCR showed that exogenous normal mitochondria of MCF-12A suppressed gene expression of glycolytic enzymes, lactate dehydrogenase A, and glucose transporter 1 and 3 of MCF-7 and MDA-MB-231 cells. Glycolysis cell-based assay revealed that normal mitochondria significantly suppressed lactate production in culture media of MCF-7, T47D, and MDA-MB-231 cells. In conclusion, normal mitochondria suppress cancer proliferation and increase drug sensitivity by the mechanism of inhibition of cancer cell glycolysis and glucose uptake.







Similar content being viewed by others
Abbreviations
- RT-qPCR:
-
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
- OXPHOS:
-
Oxidative phosphorylation
- ATP:
-
Adenosine triphosphate
- JC-1:
-
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide
- SLC2A1:
-
Glucose transporter 1
- SLC2A3:
-
Glucose transporter 3
- ALDOC:
-
Aldolase A
- ENO1:
-
Enolase 1
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GPI:
-
Glucose-6-phosphate isomerase
- HK2:
-
Hexokinase
- PFKM:
-
Phosphofructokinase-1
- PGK1:
-
Phosphoglycerate kinase 1
- PKM2:
-
Pyruvate kinase
- TMI:
-
Triosephosphate isomerase 1
- LDHA:
-
Lactate dehydrogenase A
- NADH:
-
Nicotinamide adenine dinucleotide
References
Warburg O (1965) On the origin of cancer cells. Science 123:309–314
Vender Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirement of cell proliferation. Science 324:1029–1033
Fogg VC, Lanning NJ, MacKeigan JP (2011) Mitochondria in cancer: at the crossroads of life and death. Chin J Cancer 30:526–539. doi:10.5732/cjc.011.10018
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11:325–337
Hirschhaeuser F, Sattler UGA, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71:6921–6925
Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Investig 123:3685–3692
Choi SYC, Collins CC, Gout PW, Wang Y (2013) Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol 230:350–355
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95
Elstrom R, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB (2004) Akt stimulates aerobic glycolysis in cancer cells. Caner Res 64:3892–3899
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20:51–56
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15:6479–6483
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3:177–185
Shackelford DB, Shaw RJ (2009) The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9:563–575
Elliott RL, Jiang XP, Head JF (2012) Mitochondria organelle transplantation: introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast Cancer Res Treat 136:347–354
Racker E (1974) History of the Pasteur effect and its pathobiology. Mol Cell Biochem 5:17–23
Czernin J, Phelps ME (2002) Positron emission tomography scanning: current and future application. Ann Rev Med 53:89–112
Bos R, Jacobus JM, van der Hoevenet E et al (2002) Biologic correlates of 18flurodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 20:379–387
Burt BM, Humm JL, Kooby DA et al (2001) Using positron emission tomography with [18F]EDG to predict tumor behavior in experimental colorectal cancer. Neoplasia 3:189–195
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60:916–921
Zhao Y, Butler EB, Tan M (2013) Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 4(e532):1–10. doi:10.1038/cddis.2013.60
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O et al (2010) Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitize taxol-resistant cancer cells to taxol. Mol Cancer 9:33. doi:10.1186/1476-4598-9-33
Xu RH, Pelicano H, Zhou Y et al (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65:613–621
Jang M, Kim SS, Lee J (2013) Cancer cell metabolism: implications for therapeutic targets. Exp Mol Med 45:e45. doi:10.1038/emm.2013.85
Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidemarsh DF (2004) 2-deoxy-d-glucose increases the efficacy of Adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64:31–34
Elliot RL, Barnett BG (2011) Ultrastructural observations of mitochondria in human breast carcinoma cells. Microsc Microanal 1752:194–195
Carew JS, Huang P (2002) Mitochondrial defects in cancer. Mol Cancer 1:9
Penta JS, Johnson FM, Wachsman TJ, Copeland WC (2001) Mitochondrial DNA in human malignancy. Mutat Res 488:119–133
Arnold RS, Fedewa SA, Goodman M, Osunkoya AO, Kissick HT, Morrissey C, True LD, Petros JA (2015) Bone metastasis in prostate cancer: recurring mitochondrial DNA mutation reveals selective pressure exerted by the bone microenvironment. Bone 78:81–86. doi:10.1016/j.bone.2015.04.046
Shildum A, Dornfeld K (2011) Mitochondrial amplification selectively increases doxorubicin sensitivity in breast cancer cells with acquired antiestrogen resistance. Breast Cancer Res Treat 129:785–797
Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ (2008) The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab 295:E242–E253. doi:10.1152/ajpendo.90388.2008
Kitani TL, Kami D, Matoba S, Gojo S (2014) Internalization of isolated functional mitochondria: involvement of macropinocytosis. J Cell Mol Med 18:1694–1703. doi:10.1111/jcmm.12316
Acknowledgments
This research was supported by funds from The Sallie Astor Burdine and Delta State University Foundations, Baton Rouge, Louisiana.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicting financial interest exists.
Rights and permissions
About this article
Cite this article
Jiang, XP., Elliott, R.L. & Head, J.F. Exogenous normal mammary epithelial mitochondria suppress glycolytic metabolism and glucose uptake of human breast cancer cells. Breast Cancer Res Treat 153, 519–529 (2015). https://doi.org/10.1007/s10549-015-3583-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3583-0