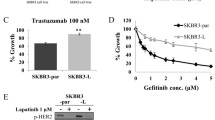
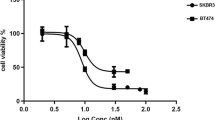
Abstract
Therapies targeting the ERBB2 receptor, including the kinase inhibitor lapatinib (Tykerb, GlaxoSmithKline), have improved clinical outcome for women with ERBB2-amplified breast cancer. However, acquired resistance to lapatinib remains a significant clinical problem, and the mechanisms governing resistance remain poorly understood. We sought to define molecular alterations that confer an acquired lapatinib resistance phenotype in ER−/ERBB2+ human breast cancer cells. ERBB2-amplified SKBR3 breast cancer cells were rendered resistant to lapatinib via culture in increasing concentrations of the drug, and molecular changes associated with a resistant phenotype were interrogated using a collaborative enzyme-enhanced immunoassay platform and immunoblotting techniques for detection of phosphorylated signaling cascade proteins. Interestingly, despite apparent inactivation of the PI3K/AKT signaling pathway, resistant cells exhibited constitutive activation of mammalian target of rapamycin complex 1 (mTORC1) and were highly sensitive to mTOR inhibition with rapamycin and the dual PI3K/mTOR inhibitor NVP-BEZ235. These data demonstrate a role for downstream activation of mTORC1 in the absence of molecular alterations leading to PI3K/AKT hyperactivation as a potential mechanism of lapatinib resistance in this model of ERBB2+ breast cancer and support the rationale of combination or sequential therapy using ERBB2 and mTOR-targeting molecules to prevent or target resistance to lapatinib. Moreover, our data suggest that assessment of mTOR substrate phosphorylation (i.e., S6) may serve as a more robust biomarker to predict sensitivity to mTOR inhibitors in the context of lapatinib resistance than PI3K mutations, loss of PTEN and p-AKT levels.





Similar content being viewed by others
Notes
CEER is a trademark of Prometheus Laboratories Inc.
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65(23):11118–11128. doi:10.1158/0008-5472.CAN-04-3841
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12(4):395–402. doi:10.1016/j.ccr.2007.08.030
Shattuck DL, Miller JK, Carraway KL III, Sweeney C (2008) Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res 68(5):1471–1477. doi:10.1158/0008-5472.CAN-07-5962
Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL (2007) Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 13(16):4909–4919. doi:10.1158/1078-0432.CCR-07-0701
O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, Crown J, O’Donovan N, Slamon DJ (2010) Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther 9(6):1489–1502. doi:10.1158/1535-7163.MCT-09-1171
Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, Pry K, Westlund R, Stein SH, Spector NL (2007) Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res 67(3):1170–1175. doi:10.1158/0008-5472.CAN-06-2101
Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, Lombardi DP, Ben Ahmed S, Citrin DL, DeSilvio ML, Harris J, Westlund RE, Salazar V, Zaks TZ, Spector NL (2008) Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol 26(7):1066–1072. doi:10.1200/JCO.2007.13.9949
Xu BH, Jiang ZF, Chua D, Shao ZM, Luo RC, Wang XJ, Liu DG, Yeo W, Yu SY, Newstat B, Preston A, Martin AM, Chi HD, Wang L (2011) Lapatinib plus capecitabine in treating HER2-positive advanced breast cancer: efficacy, safety, and biomarker results from Chinese patients. Chin J Cancer 30(5):327–335
Gayle SS, Arnold SL, O’Regan RM, Nahta R (2012) Pharmacologic inhibition of mTOR improves lapatinib sensitivity in HER2-overexpressing breast cancer cells with primary trastuzumab resistance. Anti-Cancer Agents Med Chem 12(2):151–162
Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, Baselga J (2008) Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res 68(22):9221–9230. doi:10.1158/0008-5472.CAN-08-1740
Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, Harris J, Spector NL (2006) A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Nat Acad Sci USA 103(20):7795–7800. doi:10.1073/pnas.0602468103
Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, Halsey W, Sathe GM, Martin AM, Gilmer TM (2009) Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 69(17):6871–6878. doi:10.1158/0008-5472.CAN-08-4490
Xia W, Bacus S, Husain I, Liu L, Zhao S, Liu Z, Moseley MA III, Thompson JW, Chen FL, Koch KM, Spector NL (2010) Resistance to ErbB2 tyrosine kinase inhibitors in breast cancer is mediated by calcium-dependent activation of RelA. Mol Cancer Ther 9(2):292–299. doi:10.1158/1535-7163.MCT-09-1041
Nakayama GR, Caton MC, Nova MP, Parandoosh Z (1997) Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods 204(2):205–208
Iorns E, Ward TM, Dean S, Jegg A, Thomas D, Murugaesu N, Sims D, Mitsopoulos C, Fenwick K, Kozarewa I, Naceur-Lombarelli C, Zvelebil M, Isacke CM, Lord CJ, Ashworth A, Hnatyszyn HJ, Pegram M, Lippman M (2012) Whole genome in vivo RNAi screening identifies the leukemia inhibitory factor receptor as a novel breast tumor suppressor. Breast Cancer Res Treat. doi:10.1007/s10549-012-2068-7
Kim P, Liu X, Lee T, Liu L, Barham R, Kirkland R, Leesman G, Kuller A, Ybarrondo B, Ng SC, Singh S (2011) Highly sensitive proximity mediated immunoassay reveals HER2 status conversion in the circulating tumor cells of metastatic breast cancer patients. Proteome Sci 9(1):75. doi:10.1186/1477-5956-9-75
Ward TM, Iorns E, Liu X, Hoe N, Kim P, Singh S, Dean S, Jegg AM, Gallas M, Rodriguez C, Lippman M, Landgraf R, Pegram MD (2012) Truncated p110 ERBB2 induces mammary epithelial cell migration, invasion and orthotopic xenograft formation, and is associated with loss of phosphorylated STAT5. Oncogene. doi:10.1038/onc.2012.256
Burris HA III, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O’Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF, Harris JL, Smith DA, Koch KM, Stead A, Mangum S, Spector NL (2005) Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23(23):5305–5313. doi:10.1200/JCO.2005.16.584
Jegg AWT, Issac B, Iorns E, Hoe N, Gallas M, Aparicio S, Pegram MD (2011) Identifying novel mechanisms of resistance to lapatinib in ERBB2+ breast cancer cells through whole genome mutational analysis. Paper presented at the SABCS, San Antonio
Ravichandran KS (2001) Signaling via Shc family adapter proteins. Oncogene 20(44):6322–6330. doi:10.1038/sj.onc.1204776
Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA (2003) Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem 278(12):10189–10194. doi:10.1074/jbc.M210837200
Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4(9):648–657. doi:10.1038/ncb839
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226. doi:10.1016/j.molcel.2008.03.003
Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM (2009) DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137(5):873–886. doi:10.1016/j.cell.2009.03.046
Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA (2003) Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol 5(6):566–571. doi:10.1038/ncb996
Carriere A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP (2008) Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol 18(17):1269–1277. doi:10.1016/j.cub.2008.07.078
Holz MK, Blenis J (2005) Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 280(28):26089–26093. doi:10.1074/jbc.M504045200
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66(3):1500–1508. doi:10.1158/0008-5472.CAN-05-2925
Shi Y, Yan H, Frost P, Gera J, Lichtenstein A (2005) Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 4(10):1533–1540. doi:10.1158/1535-7163.MCT-05-0068
Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM (2007) Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445(7126):437–441. doi:10.1038/nature05474
Carracedo A, Pandolfi PP (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27(41):5527–5541. doi:10.1038/onc.2008.247
Vazquez-Martin A, Oliveras-Ferraros C, Colomer R, Brunet J, Menendez JA (2008) Low-scale phosphoproteome analyses identify the mTOR effector p70 S6 kinase 1 as a specific biomarker of the dual-HER1/HER2 tyrosine kinase inhibitor lapatinib (Tykerb) in human breast carcinoma cells. Ann Oncol 19(6):1097–1109. doi:10.1093/annonc/mdm589
Acknowledgments
This study was supported by a grant from the Jiv Daya Foundation. AJ acknowledges partial support and assistance from the Sheila and David Fuente Graduate Program in Cancer Biology, Sylvester Comprehensive Cancer Center. We wish to thank Dorraya El-Ashry and Marc Lippman for critical review of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jegg, AM., Ward, T.M., Iorns, E. et al. PI3K independent activation of mTORC1 as a target in lapatinib-resistant ERBB2+ breast cancer cells. Breast Cancer Res Treat 136, 683–692 (2012). https://doi.org/10.1007/s10549-012-2252-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2252-9