Abstract
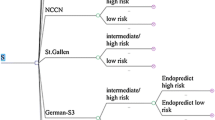
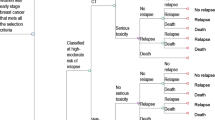
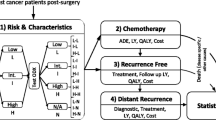
The 70-gene prognosis-signature is validated as a good predictor of recurrence for hormone receptor-positive (ER+), lymph node-negative (LN−), human epidermal growth factor receptor type 2-negative (HER2−) early stage breast cancer (ESBC) in Japanese patient population. Its high cost and potential in avoiding unnecessary adjuvant chemotherapy arouse interest in its economic impact. This study evaluates the cost-effectiveness of including the assay into Japan’s social health insurance benefit package. An economic decision tree and Markov model under Japan’s health system from the societal perspective is constructed with clinical evidence from the pool analysis of validation studies. One-way sensitivity analyses are also performed. Incremental cost-effectiveness ratio is estimated as ¥3,873,922/quality adjusted life year (QALY) (US$43,044/QALY), which is not more than the suggested social willingness-to-pay for one QALY gain from an innovative medical intervention in Japan, ¥5,000,000/QALY (US$55,556/QALY). However, sensitivity analyses show the instability of this estimation. The introduction of the assay into Japanese practice of ER+, LN−, HER2− ESBC treatment by including it to Japan’s social health insurance benefit package has a reasonable chance to be judged as cost-effective and may be justified as an efficient deployment of finite health care resources.

Similar content being viewed by others
References
The Japanese Breast Cancer Society (2010) National breast cancer registry report—provisional edition no. 39 2008 cases. The Japanese Breast Cancer Society, Tokyo (in Japanese)
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and endocrine therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, Panel Members (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol 20(8):1319–1329
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415(6871):530–536
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347(25):1999–2009
Buyse M, Loi S, van’t Veer L, Viale G, Delorenzi M, Glas AM, d’Assignies MS, Bergh J, Lidereau R, Ellis P, Harris A, Bogaerts J, Therasse P, Floore A, Amakrane M, Piette F, Rutgers E, Sotiriou C, Cardoso F, Piccart MJ, TRANSBIG Consortium (2006) Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 98(17):1183–1192
Bueno-de-Mesquita JM, Linn SC, Keijzer R, Wesseling J, Nuyten DS, van Krimpen C, Meijers C, de Graaf PW, Bos MM, Hart AA, Rutgers EJ, Peterse JL, Halfwerk H, de Groot R, Pronk A, Floore AN, Glas AM, Van’t Veer LJ, van de Vijver MJ (2009) Validation of 70-gene prognosis signature in node-negative breast cancer. Breast Cancer Res Treat 117(3):483–495
Ishitobi M, Goranova TE, Komoike Y, Motomura K, Koyama H, Glas AM, van Lienen E, Inaji H, Van’t Veer LJ, Kato K (2010) Clinical utility of the 70-gene MammaPrint profile in a Japanese population. Jpn J Clin Oncol 40(6):508–512
de Snoo F, Bender R, Glas A, Rutgers E (2009) Gene expression profiling: decoding breast cancer. Surg Oncol 18(4):366–378
Retèl VP, Joore MA, Knauer M, Linn SC, Hauptmann M, Harten WH (2010) Cost-effectiveness of the 70-gene signature versus St. Gallen guidelines and Adjuvant Online for early breast cancer. Eur J Cancer 46(8):1382–1391
Chen E, Tong KB, Malin JL (2010) Cost-effectiveness of 70-gene MammaPrint signature in node-negative breast cancer. Am J Manag Care 16(12):e333–e342
Kondo M, Hoshi SL, Yamanaka T, Ishiguro H, Toi M (2011) Economic evaluation of the 21-gene signature (Oncotype DX®) in lymph node-negative/positive, hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03). Breast Cancer Res Treat 127(3):739–749
Kondo M, Hoshi SL, Ishiguro H, Yoshibayashi H, Toi M (2008) Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat 112(1):175–187
Iwata H, Saeki T (2006) Current practices in breast cancer treatment in Japan—a questionnaire survey. Jpn J Breast Cancer 21(3):311–322
Japanese Breast Cancer Society (2010) Evidence-based breast cancer care guideline: 1 drug treatments 2010 version. Kanehara Shuppan, Tokyo (in Japanese)
Hornberger J, Cosler LE, Lyman GH (2005) Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 11(5):313–324
Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC (2004) HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 22(5):854–863
Ministry of Health, Labour and Welfare (2007) The 20th life tables. Health and Welfare Statistics Association, Tokyo
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21(11):2101–2109
Hillner BE, Smith TJ (1991) Efficacy and cost effectiveness of adjuvant chemotherapy in women with node-negative breast cancer. A decision-analysis model. N Engl J Med 324(3):160–168
Earle CC, Chapman RH, Baker CS, Bell CM, Stone PW, Sandberg EA, Neumann PJ (2000) Systematic overview of cost-utility assessments in oncology. J Clin Oncol 18(18):3302–3317
Cole BF, Gelber RD, Gelber S, Coates AS, Goldhirsch A (2001) Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet 358(9278):277–286
Gold MR, Siegel JE, Russell LB, Weinstein MC (eds) (1996) Cost-effectiveness in health and medicine. Oxford University Press, New York
Japan Society of Clinical Oncology (2005) Guideline of appropriate use of anti cancer drugs: breast cancer. Int J Clin Oncol 10:15–55 (in Japanese)
Iwata H, Nakamura S, Toi M, Shin E, Masuda N, Ohno S, Takatsuka Y, Hisamatsu K, Yamazaki K, Kusama M, Kaise H, Sato Y, Kuroi K, Akiyama F, Tsuda H, Kurosumi M (2005) Interim analysis of a phase II trial of cyclophosphamide, epirubicin and 5-fluorouracil (CEF) followed by docetaxel as preoperative chemotherapy for early stage breast carcinoma. Breast Cancer 12(2):99–103
Papaldo P, Ferretti G, Di Cosimo S, Giannarelli D, Marolla P, Lopez M, Cortesi E, Antimi M, Terzoli E, Carlini P, Vici P, Botti C, Di Lauro L, Naso G, Nisticò C, Mottolese M, Di Filippo F, Ruggeri EM, Ceribelli A, Cognetti F (2006) Does granulocyte colony-stimulating factor worsen anemia in early breast cancer patients treated with epirubicin and cyclophosphamide? J Clin Oncol 24(19):3048–3055
Kondo M, Hoshi SL, Toi M (2009) Economic evaluation of chemoprevention of breast cancer with tamoxifen and raloxifene among high-risk women in Japan. Br J Cancer 100(2):281–290
Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K (2010) International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 19(4):422–437
Drummond M, Pang F (2001) Transferability of economic evaluation results. In: Drummond M, McGuire A (eds) Economic evaluation in health care: merging theory with practice. Oxford University Press, Oxford, pp 256–276
Goeree R, He J, O’Reilly D, Tarride JE, Xie F, Lim M, Burke N (2011) Transferability of health technology assessments and economic evaluations: a systematic review of approaches for assessment and application. Clinicoecon Outcomes Res 3:89–104
Ishiguro H, Kondo M, Hoshi SL, Takada M, Nakamura S, Teramukai S, Yanagihara K, Toi M (2010) Economic evaluation of intensive chemotherapy with prophylactic granulocyte colony-stimulating factor for patients with high-risk early breast cancer in Japan. Clin Ther 32(2):311–326
Weigel MT, Dowsett M (2010) Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer 17(4):R245–R262
Akaza H, Hill D, Roh JK, Hao XS (2010) Proposal on the establishment of infrastructure for providing cancer treatment in Asia in the context of global health: Asia–Pacific cancer conference (12–14 November 2009). Jpn J Clin Oncol 40(Suppl 1):i86–i92
Shiroiwa T, Fukuda T, Shimozuma K, Ohashi Y, Tsutani K (2008) The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat 109(3):559–566
Acknowledgments
The study was funded by Japan’s Ministry of Health, Labour and Welfare research grant, ‘Reduction and lowering of recurrence risk, toxicity and pharmacoeconomic cost by prediction of efficacy for anti-cancer agents in breast cancer patients’, led by Masakazu Toi (H22-GANRINSHO-IPPAN-039), and was also supported by Grant-in-Aid for Scientific Research (C) by Japan’s Ministry of Education, Culture, Sports, Science and Technology (No. 22590451).
Conflict of interest
All authors declare that there is no possible conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondo, M., Hoshi, SL., Ishiguro, H. et al. Economic evaluation of the 70-gene prognosis-signature (MammaPrint®) in hormone receptor-positive, lymph node-negative, human epidermal growth factor receptor type 2-negative early stage breast cancer in Japan. Breast Cancer Res Treat 133, 759–768 (2012). https://doi.org/10.1007/s10549-012-1979-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-1979-7