Abstract
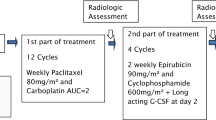
Every-2-week (dose-dense) adjuvant doxorubicin (A) plus cyclophosphamide (C) followed by cremophor-formulated paclitaxel (cf-P) was efficacious in metastatic breast cancer (BC). Albumin-bound paclitaxel (ab-P) was safe and more effective than cf-P, and the addition of bevacizumab to cf-P improved efficacy. This study compared the safety of dose-dense ab-P vs cf-P plus bevacizumab following dose-dense adjuvant AC for early-stage BC. Patients and Methods: Women with operable, histologically confirmed BC were randomized to 4 cycles of dose-dense A 60 mg/m2 plus C 600 mg/m2 IV with SC pegfilgrastim, followed by 4 cycles of either dose-dense IV ab-P 260 mg/m2 or cf-P 175 mg/m2. Bevacizumab was given during and following chemotherapy. 97 and 96% of patients completed 4 cycles of AC therapy, while 84 and 85% of patients completed 4 cycles of taxane therapy in the ab-P and cf-P arms, respectively (N = 197). Baseline patient characteristics were similar. The most common grade ≥3 taxane-related adverse events (AEs) were fatigue and neutropenia. Dose reductions were similar between the treatment arms. During AC therapy, the majority of dose reductions were due to febrile neutropenia; during taxane therapy, the majority of cases were due to neuropathy. No taxane-related dose interruption occurred in the ab-P arm, while 3 occurred in the cf-P arm due to hypersensitivity reactions. The mean cumulative paclitaxel dose was 950.5 and 660.8 mg/m2 in the ab-P and cf-P arms, respectively. A 44% higher paclitaxel dose was delivered in the ab-P compared with the cf-P arm (P < 0.0001), while achieving a similar safety profile. ab-P plus bevacizumab following AC therapy without prophylactic premedications was tolerable in early-stage BC patients.


Similar content being viewed by others
References
Campone M, Fumoleau P, Bourbouloux E et al (2005) Taxanes in adjuvant breast cancer setting: which standard in Europe? Crit Rev Oncol Hematol 55:167–175
Citron ML, Berry DA, Cirrincione C et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431–1439
Hayes DF, Thor A, Dressler L et al. (2006) HER2 predicts benefit from adjuvant paclitaxel after AC in node-positive breast cancer: CALGB 9344. J Clin Oncol (Meeting Abstracts) 24:Abstr#510
Henderson IC, Bhatia V (2007) Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert Rev Anticancer Ther 7:919–943
Ibrahim NK, Desai N, Legha S et al (2002) Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res 8:1038–1044
Gradishar WJ, Tjulandin S, Davidson N et al (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23:7794–7803
Robert N, Krekow L, Stokoe C et al (2011) Adjuvant dose-dense doxorubicin plus cyclophosphamide followed by dose-dense nab-paclitaxel is safe in women with early-stage breast cancer: a pilot study. Breast Cancer Res Treat 125:115–120
Volk LD, Flister MJ, Bivens CM et al (2008) Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia 10:613–623
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Bria E, Nistico C, Cuppone F et al (2006) Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer 106:2337–2344
De Laurentiis M, Cancello G, D’Agostino D et al (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26:44–53
Ferguson T, Wilcken N, Vagg R et al (2007) Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev:CD004421
Henderson IC, Berry DA, Demetri GD et al (2003) Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976–983
Kahan Z, Uhercsak G, Hajnal-Papp R et al (2005) Dose-dense sequential adriamycin-Paclitaxel-cyclophosphamide chemotherapy is well tolerated and safe in high-risk early breast cancer. Oncology 68:446–453
Sachdev JC, Jahanzeb M (2008) Evolution of bevacizumab-based therapy in the management of breast cancer. Clin Breast Cancer 8:402–410
Arnedos M, Sutherland S, Ashley S et al (2008) Routine prophylactic granulocyte colony stimulating factor (GCSF) is not necessary with accelerated (dose dense) paclitaxel for early breast cancer. Breast Cancer Res Treat 112:1–4
Sugarman S, Wasserheit C, Hodgman E et al (2009) A pilot study of dose-dense adjuvant paclitaxel without growth factor support for women with early breast carcinoma. Breast Cancer Res Treat 115:609–612
Acknowledgment
This study was sponsored by Abraxis BioScience, a wholly-owned subsidiary of Celgene. Medical writing assistance was provided by Anita Schmid, PhD, Celgene.
Conflict of interest
The other authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pippen, J., Paul, D., Vukelja, S. et al. Dose-dense doxorubicin and cyclophosphamide followed by dose-dense albumin-bound paclitaxel plus bevacizumab is safe as adjuvant therapy in patients with early stage breast cancer. Breast Cancer Res Treat 130, 825–831 (2011). https://doi.org/10.1007/s10549-011-1678-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1678-9