Abstract
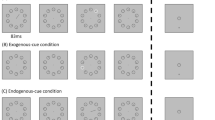
Planet Earth’s motion yields a 50 % day–50 % night yearly balance in every latitude or longitude, so survival must be guaranteed in very different light conditions in many species, including human. Cone- and rod-dominant vision, respectively specialized in light and darkness, present several processing differences, which are—at least partially—reflected in event-related potentials (ERPs). The present experiment aimed at characterizing exogenous attention to threatening (spiders) and neutral (wheels) distractors in two environmental light conditions, low mesopic (L, 0.03 lx) and high mesopic (H, 6.5 lx), yielding a differential photoreceptor activity balance: rod > cone and rod < cone, respectively. These distractors were presented in the lower visual hemifield while the 40 participants were involved in a digit categorization task. Stimuli, both targets (digits) and distractors, were exactly the same in L and H. Both ERPs and behavioral performance in the task were recorded. Enhanced attentional capture by salient distractors was observed regardless of ambient light level. However, ERPs showed a differential pattern as a function of ambient light. Thus, significantly enhanced amplitude to salient distractors was observed in posterior P1 and early anterior P2 (P2a) only during the H context, in late P2a during the L context, and in occipital P3 during both H and L contexts. In other words, while exogenous attention to threat was equally efficient in light and darkness, cone-dominant exogenous attention was faster than rod-dominant, in line with previous data indicating slower processing times for rod- than for cone-dominant vision.


Similar content being viewed by others
Notes
The conspicuous N2 at occipital sites was also analyzed despite the lack of an effect of Distractor in grand averages (and hence falling outside the scope of this study). Effects were non-significant: F(1,39) < 1 and p > 0.4 in all relevant contrasts: Light, Distractor, LightxDistractor, Electrodes(O1, Oz, O2)xLightxDistractor.
References
Benedek G, Benedek K, Kéri S, Letoha T, Janáky M (2003) Human scotopic spatiotemporal sensitivity: a comparison of psychophysical and electrophysiological data. Doc Ophthalmol 106:201–207
Carretié L (2014) Exogenous (automatic) attention to emotional stimuli: a review. Cogn Affect Behav Neurosci 14:1228–1258
Carretié L, Hinojosa JA, Martín-Loeches M, Mercado F, Tapia M (2004) Automatic attention to emotional stimuli: neural correlates. Hum Brain Mapp 22:290–299
Carretié L, Hinojosa JA, Mercado F, Tapia M (2005) Cortical response to subjectively unconscious danger. Neuroimage 24:615–623
Carretié L, Hinojosa JA, López-Martín S, Albert J, Tapia M, Pozo MA (2009) Danger is worse when it moves: neural and behavioral indices of enhanced attentional capture by dynamic threatening stimuli. Neuropsychologia 47:364–369
Carretié L, Ruiz-Padial E, López-Martín S, Albert J (2011) Decomposing unpleasantness: differential exogenous attention to disgusting and fearful stimuli. Biol Psychol 86:247–253
Carretié L, Albert J, López-Martín S, Hoyos S, Kessel D, Tapia M, Capilla A (2013a) Differential neural mechanisms underlying exogenous attention to peripheral and central distracters. Neuropsychologia 51:1838–1847
Carretié L, Kessel D, Carboni A, López-Martín S, Albert J, Tapia M, Mercado F, Capilla A, Hinojosa JA (2013b) Exogenous attention to facial versus non-facial emotional visual stimuli. Soc Cogn Affect Neurosci 8:764–773
Carretié L, Ruiz-Padial E, Mendoza MT (2015) An event-related potential study on the interaction between lighting level and stimulus spatial location. Front Hum Neurosci 9:637
Cohn R, Hurley CW (1985) Differential visual evoked cortical responses to direct and peripheral stimulation in man. Electroencephalogr Clin Neurophysiol 61:157–160
Curcio CA, Sloan KR, Kalina RE, Hendrickson AE (1990) Human photoreceptor topography. J Comp Neurol 292:497–523
Delplanque S, Silvert L, Hot P, Sequeira H (2005) Event-related P3a and P3b in response to unpredictable emotional stimuli. Biol Psychol 68:107–120
Ellemberg D, Hammarrenger B, Lepore F, Roy M, Guillemot J (2001) Contrast dependency of VEPs as a function of spatial frequency: the parvocellular and magnocellular contributions to human VEPs. Spat Vis 15:99–111
Feng C, Wang L, Wang N, Gu R, Luo Y (2012) The time course of implicit processing of erotic pictures: an event-related potential study. Brain Res 1489:48–55
Franconeri SL, Simons DJ (2003) Moving and looming stimuli capture attention. Percept Psychophys 65:999–1010
Franconeri SL, Simons DJ (2005) The dynamic events that capture visual attention: a reply to Abrams and Christ (2005) Percept. Psychophys 67:962–966
Hammarrenger B, Roy M, Ellemberg D, Labrosse M, Orquin J, Lippe S, Lepore F (2007) Developmental delay and magnocellular visual pathway function in very-low-birthweight preterm infants. Dev Med Child Neurol 49:28–33
Holmes A, Kiss M, Eimer M (2006) Attention modulates the processing of emotional expression triggered by foveal faces. Neurosci Lett 394:48–52
Hopfinger JB, Mangun GR (2001) Electrophysiological studies of reflexive attention. In: Folk CL, Gibson BS (eds) Attraction, distraction and action: multiple perspectives on attentional capture. Elsevier, New York, pp 3–26
Ives HE (1922) Critical frequency relations in scotopic vision. J Opt Soc Am 6:254–267
Jonas JB, Schneider U, Naumann GO (1992) Count and density of human retinal photoreceptors. Graef Arch Clin Exp 230:505–510
Jung TP, Makeig S, Humphries C, Lee TW, Mckeown MJ, Iragui V, Sejnowski TJ (2000) Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37:163–178
Junhong H, Renlai Z, Senqi H (2013) Effects on automatic attention due to exposure to pictures of emotional faces while performing Chinese word judgment tasks. PLoS One 8(10):e75386
Kawamura S, Tachibanaki S (2008) Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp Biochem Physiol A 150:369–377
Kilavik BE, Kremers J (2001) Rod and L-cone interactions in a deuteranope at different temporal frequencies. Color Res Appl 26(S1):S76–S78
Kim EY, Lee S, Park G, Kim S, Kim I, Chae J, Kim HT (2013) Gender difference in event related potentials to masked emotional stimuli in the oddball task. Psychiatr Investig 10:164–172
Laycock R, Crewther SG (2008) Towards an understanding of the role of the ‘magnocellular advantage’ in fluent reading. Neurosci Biobehav Rev 32:1494–1506
Lee BB, Smith VC, Pokorny J, Kremers J (1997) Rod inputs to macaque ganglion cells. Vis Res 37:2813–2828
Livingstone MS, Hubel DH (1987) Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci 7:3416–3468
Luck SJ (1994) Electrophysiological correlates of feature analysis during visual search. Psychophysiology 31:291–308
Luck SJ (2012) Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related electrophysiological correlates. In: Luck SJ, Kappenman ES (eds) The Oxford handbook of event-related potential components. Oxford University Press, Oxford, pp 329–360
MacLeod DI (1972) Rods cancel cones in flicker. Nature 235:173–174
Masland RH (2001) The fundamental plan of the retina. Nat Neurosci 4:877–886
Maunsell JHR, Ghose GM, Assad JA, McAdams CJ, Boudreau CE, Noerager BD (1999) Visual response latencies of magnocellular and parvocellular LGN neurons in macaque monkeys. Vis Neurosci 16:1–14
Münch M, Plomp G, Thunell E, Kawasaki A, Scartezzini J, Herzog MH (2014) Different colors of light lead to different adaptation and activation as determined by high-density EEG. NeuroImage 101:547–554
Narisada K, Schreuder D (2004) Light pollution handbook. Springer, New York
Nunez PL, Srinivasan R (2006) Electric fields of the brain: the neurophysics of EEG. Oxford University Press, Oxford
Parisi V, Ziccardi L, Stifano G, Montrone L, Gallinaro G, Falsini B (2010) Impact of regional retinal responses on cortical visually evoked responses: multifocal ERGs and VEPs in the retinitis pigmentosa model. Clin Neurophysiol 121:380–385
Perry V, Oehler R, Cowey A (1984) Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience 12:1101–1123
Purpura K, Kaplan E, Shapley RM (1988) Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci USA 85:4534–4537
Rudvin I, Valberg A (2006) Flicker VEPs reflecting multiple rod and cone pathways. Vis Res 46:699–717
Schreuder D (2008) Outdoor lighting: physics, vision and perception. Springer, New York
Sharpe LT, Stockman A, MacLeod DIA (1989) Rod flicker perception: scotopic duality, phase lags and destructive interference. Vis Res 29:1539–1559
Stanford MS, Vasterling JJ, Mathias CW, Constans JI, Houston RJ (2001) Impact of threat relevance on P3 event-related potentials in combat-related post-traumatic stress disorder. Psychiatr Res 102:125–137
Sterling P (2003) How retinal circuits optimize the transfer of visual information. In: Chalupa L, Werner J (eds) The visual neurosciences. MIT Press, Cambridge, pp 234–259
Stockman A, Sharpe LT (2006) Into the twilight zone: the complexities of mesopic vision and luminous efficiency. Ophthalmic Physiol Opt 26:225–239
Sun H, Pokorny J, Smith VC (2001) Brightness induction from rods. J Vis 1:32–41
Vuilleumier P, Armony JL, Driver J, Dolan RJ (2003) Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci 6:624
Wässle H (2004) Parallel processing in the mammalian retina. Nat Rev Neurosci 5:747–757
Zele AJ, Cao D (2014) Vision under mesopic and scotopic illumination. Front Psychol 5:1594
Acknowledgments
This research was supported by the Grants PSI2014-54853-P and PSI2012-37090 from the Ministerio de Economía y Competitividad of Spain (MINECO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carretié, L., Ruiz-Padial, E. Ambient Light Modulation of Exogenous Attention to Threat. Brain Topogr 29, 847–855 (2016). https://doi.org/10.1007/s10548-016-0510-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-016-0510-6