Abstract
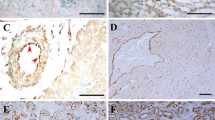
Fabry disease is caused by deficient activity of α-galactosidase A and subsequent intracellular accumulation of glycosphingolipids, mainly globotriaosylceramide (Gb3). Vascular endothelial cells may play important roles in disease pathogenesis, and are one of the main target cell types in therapeutic interventions. In this study, we generated immortalized aortic endothelial cell lines from a mouse model of Fabry disease. These cells retained endothelial cell-specific markers and functions. Gb3 expression level in one of these clones (referred to as FMEC2) was highly susceptible to culture media, and appeared to be regulated by glucosylceramide synthase. Results also showed that Gb3 could be upregulated by hydrocortisone. FMEC2 express the mannose 6-phosphate receptor and sortilin but not the mannose receptor. Uptake studies suggested that sortilin plays a role in the binding and internalization of mammalian cell-produced α-galactosidase A. Moss-aGal (a plant-made enzyme) was endocytosed by FMEC2 via a receptor other than the aforementioned receptors. In conclusion, this study suggests that glucosylceramide synthase and hydrocortisone may play important roles in modulating Gb3 levels in Fabry mouse aortic endothelial cells, and that endocytosis of recombinant α-galactosidase A involves a combination of multiple receptors depending on the properties of the enzyme.



Similar content being viewed by others
References
Aerts JM, Groener JE, Kuiper S et al (2008) Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A 105:2812–2817
Altarescu G, Moore DF, Pursley R et al (2001) Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke 32:1559–1562
Bangari DS, Ashe KM, Desnick RJ et al (2015) alpha-Galactosidase A knockout mice: progressive organ pathology resembles the type 2 later-onset phenotype of Fabry disease. Am J Pathol 185:651–665
Brady R, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L (1967) Enzymatic defect in Fabry disease: ceramide trihexosidase deficiency. N Engl J Med 276:1163–1167
Bu G, Marzolo MP (2000) Role of rap in the biogenesis of lipoprotein receptors. Trends Cardiovasc Med 10:148–155
Cabrera-Salazar MA, Deriso M, Bercury SD et al (2012) Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease. PLoS One 7:e43310
Christensen EI, Zhou Q, Sorensen SS et al (2007) Distribution of alpha-galactosidase A in normal human kidney and renal accumulation and distribution of recombinant alpha-galactosidase A in Fabry mice. J Am Soc Nephrol 18:698–706
Coutinho MF, Prata MJ, Alves S (2012) A shortcut to the lysosome: the mannose-6-phosphate-independent pathway. Mol Genet Metab 107:257–266
Desnick RJ, Ioannou YA, Eng CM (2001) a-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 3733–3774
Futerman AH, Pagano RE (1991) Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J 280(Pt 2):295–302
Heare T, Alp NJ, Priestman DA et al (2007) Severe endothelial dysfunction in the aorta of a mouse model of Fabry disease; partial prevention by N-butyldeoxynojirimycin treatment. J Inherit Metab Dis 30:79–87
Heuser JE, Anderson RG (1989) Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol 108:389–400
Hu F, Padukkavidana T, Vaegter CB et al (2010) Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68:654–667
Kang JJ, Shu L, Park JL, Shayman JA, Bodary PF (2014) Endothelial nitric oxide synthase uncoupling and microvascular dysfunction in the mesentery of mice deficient in alpha-galactosidase A. Am J Physiol Gastrointest Liver Physiol 306:G140–G146
Larsen JV, Hansen M, Moller B et al (2010) Sortilin facilitates signaling of ciliary neurotrophic factor and related helical type 1 cytokines targeting the gp130/leukemia inhibitory factor receptor beta heterodimer. Mol Cell Biol 30:4175–4187
Marchesan D, Cox TM, Deegan PB (2012) Lysosomal delivery of therapeutic enzymes in cell models of Fabry disease. J Inherit Metab Dis 35:1107–1117
Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD (1994) The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77:579–586
Moore DF, Scott LT, Gladwin MT et al (2001) Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation 104:1506–1512
Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM (1999) Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem 274:8832–8836
Nielsen MS, Madsen P, Christensen EI et al (2001) The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J 20:2180–2190
Nishie T, Hikimochi Y, Zama K et al (2010) Beta4-galactosyltransferase-5 is a lactosylceramide synthase essential for mouse extra-embryonic development. Glycobiology 20:1311–1322
Nykjaer A, Lee R, Teng KK et al (2004) Sortilin is essential for proNGF-induced neuronal cell death. Nature 427:843–848
Ohshima T, Murray GJ, Swaim WD et al (1997) alpha-Galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci U S A 94:2540–2544
Park JL, Whitesall SE, D’Alecy LG, Shu L, Shayman JA (2008) Vascular dysfunction in the alpha-galactosidase A-knockout mouse is an endothelial cell-, plasma membrane-based defect. Clin Exp Pharmacol Physiol 35:1156–1163
Petersen CM, Nielsen MS, Nykjaer A et al (1997) Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem 272:3599–3605
Prabakaran T, Nielsen R, Larsen JV et al (2011) Receptor-mediated endocytosis of alpha-galactosidase A in human podocytes in Fabry disease. PLoS One 6:e25065
Prabakaran T, Nielsen R, Satchell SC et al (2012) Mannose 6-phosphate receptor and sortilin mediated endocytosis of alpha-galactosidase A in kidney endothelial cells. PLoS One 7:e39975
Schiffmann R (2009) Fabry disease. Pharmacol Ther 122:65–77
Shen JS, Meng XL, Schiffmann R, Brady RO, Kaneski CR (2007) Establishment and characterization of Fabry disease endothelial cells with an extended lifespan. Mol Genet Metab 92:137–144
Shen JS, Meng XL, Wight-Carter M et al (2015a) Blocking hyperactive androgen receptor signaling ameliorates cardiac and renal hypertrophy in Fabry mice. Hum Mol Genet 24:3181–3191
Shen JS, Busch A, Day TS, et al (2015b) Mannose receptor-mediated delivery of moss-made alpha-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J Inherit Metab Dis doi:10.1007/s10545-015-9886-9
Shu L, Murphy HS, Cooling L, Shayman JA (2005) An in vitro model of Fabry disease. J Am Soc Nephrol 16:2636–2645
Sillence DJ, Puri V, Marks DL et al (2002) Glucosylceramide modulates membrane traffic along the endocytic pathway. J Lipid Res 43:1837–1845
Sorvillo N, Pos W, van den Berg LM et al (2012) The macrophage mannose receptor promotes uptake of ADAMTS13 by dendritic cells. Blood 119:3828–3835
Takahashi K, Donovan MJ, Rogers RA, Ezekowitz RA (1998) Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res 292:311–323
Troussard AA, Khallou J, Mann CJ et al (1995) Inhibitory effect on the lipolysis-stimulated receptor of the 39-kDa receptor-associated protein. J Biol Chem 270:17068–17071
Wagner DD, Olmsted JB, Marder VJ (1982) Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol 95:355–360
Acknowledgments
We thank Dilechia Hawthorne (Institute of Metabolic Disease, Dallas) for her technical assistance during the study. This work was supported by Baylor Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xing-Li Meng, Taniqua S. Day, Nathan McNeill, Paula Ashcraft, Zhi-Ping Liu, and Jin-Song Shen declare that they have no conflict of interest. Thomas Frischmuth is employee in Greenovation Biotech. Seng H. Cheng is employee in Genzyme. Raphael Schiffmann received research funds and honoraria in the past 5 years from Genzyme, Shire and Amicus Therapeutics.
Additional information
Communicated by: Robin Lachmann
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 724 kb)
Rights and permissions
About this article
Cite this article
Meng, XL., Day, T.S., McNeill, N. et al. Molecular basis for globotriaosylceramide regulation and enzyme uptake in immortalized aortic endothelial cells from Fabry mice. J Inherit Metab Dis 39, 447–455 (2016). https://doi.org/10.1007/s10545-016-9920-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-016-9920-6