Abstract
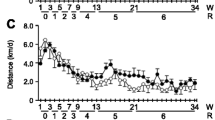
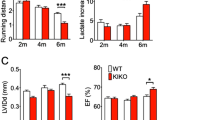
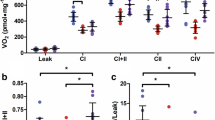
Barth syndrome (BTHS) is an X-linked metabolic disorder that causes cardiomyopathy in infancy and is linked to mutations within the Tafazzin (TAZ) gene. The first mouse model, a TAZ knockdown model (TAZKD), has been generated to further understand the bioenergetics leading to cardiomyopathy. However, the TAZKD model does not show early signs of cardiomyopathy, and cardiac pathophysiology has not been documented until 7–8 months of age. Here we sought to determine the impact of endurance training on the cardiac and skeletal muscle phenotype in young TAZKD mice. TAZKD exercise trained (TAZKD-ET) and control exercise trained (CON-ET) mice underwent a 35-day swimming protocol. Non-trained aged matched TAZKD and CON mice were used as controls. At the end of the protocol, cardiac MRI was used to assess cardiac parameters. Cardiac MRI showed that training resulted in cardiac hypertrophy within both groups and did not result in a decline of ejection fraction. TAZKD mice exhibited a decrease in respiratory complex I, III, and IV enzymatic activity in cardiac tissue compared to control mice; however, training led to an increase in complex III activity in TAZKD-ET mice resulting in similar levels to those of CON-ET mice. 31P magnetic resonance spectroscopy of the gastrocnemius showed a significantly lowered pH in TAZKD-ET mice post electrical-stimulation compared to CON-ET mice. Endurance training does not accelerate cardiac dysfunction in young TAZKD mice, but results in beneficial physiological effects. Furthermore, our results suggest that a significant drop in intracellular pH levels may contribute to oxidative phosphorylation defects during exercise.



Similar content being viewed by others
References
Acehan D, Vaz F, Houtkooper RH et al (2011) Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286:899–908
Barth PG, Van den Bogert C, Bolhuis PA et al (1996) X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): respiratory-chain abnormalities in cultured fibroblasts. J Inherit Metab Dis 19:157–160
Bostrom P, Mann N, Wu J et al (2010) C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143:1072–1083
Chance B, Im J, Nioka S et al (2006) Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR Biomed 19:904–926
Christodoulou J, McInnes RR, Jay V et al (1994) Barth syndrome: clinical observations and genetic linkage studies. Am J Med Genet 50:255–264
Elliott MA, Walter GA, Swift A et al (1999) Spectral quantitation by principal component analysis using complex singular value decomposition. Magn Reson Med 41:450–455
Evangelista FS, Brum PC, Krieger JE (2003) Duration-controlled swimming exercise training induces cardiac hypertrophy in mice. Braz J Med Biol Res 36:1751–1759
Forbes SC, Paganini AT, Slade JM et al (2009) Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol 296:R161–R170
He Q (2010) Tafazzin knockdown causes hypertrophy of neonatal ventricular myocytes. Am J Physiol Heart Circ Physiol 299:H210–H216
He Q, Wang M, Harris N et al (2013) Tafazzin knockdown interrupts cell cycle progression in cultured neonatal ventricular fibroblasts. Am J Physiol Heart Circ Physiol 305:H1332–H1343
Houtkooper RH, Turkenburg M, Poll-The BT et al (2009) The enigmatic role of tafazzin in cardiolipin metabolism. Biochim Biophys Acta 1788:2003–2014
Jeppesen TD, Schwartz M, Olsen DB et al (2003) Oxidative capacity correlates with muscle mutation load in mitochondrial myopathy. Ann Neurol 54:86–92
Jeppesen TD, Schwartz M, Olsen DB et al (2006) Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 129:3402–3412
Jubrias SA, Crowther GJ, Shankland EG et al (2003) Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol 553:589–599
Kaplan ML, Cheslow Y, Vikstrom K et al (1994) Cardiac adaptations to chronic exercise in mice. Am J Physiol 267:H1167–H1173
Kiebish MA, Yang K, Liu X et al (2013) Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J Lipid Res 54:1312–1325
Kirkinezos IG, Moraes CT (2001) Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol 12:449–457
Malhotra A, Xu Y, Ren M et al (2009) Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim Biophys Acta 1791:314–320
Phoon CK, Acehan D, Schlame M et al (2012) Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. J Am Heart Assoc 1
Poburko D, Santo-Domingo J, Demaurex N (2011) Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J Biol Chem 286:11672–11684
Powers C, Huang Y, Strauss A et al (2013) Diminished exercise capacity and mitochondrial bc1 complex deficiency in Tafazzin-knockdown mice. Front Physiol 4:74
Radak Z, Zhao Z, Koltai E et al (2013) Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal 18:1208–1246
Soustek MS, Falk DJ, Mah CS et al (2011) Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum Gene Ther 22:865–871
Spencer CT, Bryant RM, Day J et al (2006) Cardiac and clinical phenotype in Barth syndrome. Pediatrics 118:e337–e346
Spencer CT, Byrne BJ, Bryant RM et al (2011) Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. Am J Physiol Heart Circ Physiol 301:H2122–H2129
Spinazzi M, Casarin A, Pertegato V et al (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7:1235–1246
Taivassalo T, Jensen TD, Kennaway N et al (2003) The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain 126:413–423
Taylor DJ, Bore PJ, Styles P et al (1983) Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med 1:77–94
Walter G, Vandenborne K, Elliott M et al (1999) In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol 519(Pt 3):901–910
Compliance with Ethics Guidelines
Conflict of Interest
None.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Verena Peters
Rights and permissions
About this article
Cite this article
Soustek, M.S., Baligand, C., Falk, D.J. et al. Endurance training ameliorates complex 3 deficiency in a mouse model of Barth syndrome. J Inherit Metab Dis 38, 915–922 (2015). https://doi.org/10.1007/s10545-015-9834-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-015-9834-8