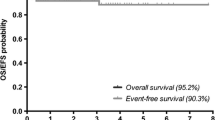
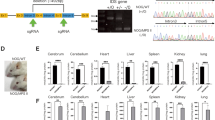
Abstract
Mucopolysaccharidosis type II (MPS II) is a lysosomal storage disorder caused by deficient activity of the iduronate-2-sulfatase. This leads to accumulation of glycosaminoglycans (GAGs) in the lysosomes of various cells. Although it has been proposed that bone marrow transplantation (BMT) may have a beneficial effect for patients with MPS II, the requirement for donor-cell chimerism to reduce GAG levels is unknown. To address this issue, we transplanted various ratios of normal and MPS II bone marrow cells in a mouse model of MPS II and analyzed GAG accumulation in various tissues. Chimerism of whole leukocytes and each lineage of BMT recipients’ peripheral blood was similar to infusion ratios. GAGs were significantly reduced in the liver, spleen, and heart of recipients. The level of GAG reduction in these tissues depends on the percentage of normal-cell chimerism. In contrast to these tissues, a reduction in GAGs was not observed in the kidney and brain, even if 100 % donor chimerism was achieved. These observations suggest that a high degree of chimerism is necessary to achieve the maximum effect of BMT, and donor lymphocyte infusion or enzyme replacement therapy might be considered options in cases of low-level chimerism in MPS II patients.




Similar content being viewed by others
References
Akiyama K, Shimada Y, Higuchi T et al (2014) Enzyme augmentation therapy enhances the therapeutic efficacy of bone marrow transplantation in mucopolysaccharidosis type II mice. Mol Genet Metab 111:139–146
Araya K, Sakai N, Mohri I et al (2009) Localized donor cells in brain of a Hunter disease patient after cord blood stem cell transplantation. Mol Genet Metab 98:255–263
Boelens JJ, Aldenhoven M, Purtill D et al (2013) Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood 121:3981–3987
Burton BK, Whiteman DA, Investigators HOS (2011) Incidence and timing of infusion-related reactions in patients with mucopolysaccharidosis type II (Hunter syndrome) on idursulfase therapy in the real-world setting: a perspective from the Hunter Outcome Survey (HOS). Mol Genet Metab 103:113–120
Enquist IB, Nilsson E, Mansson JE, Ehinger M, Richter J, Karlsson S (2009) Successful low-risk hematopoietic cell therapy in a mouse model of type 1 Gaucher disease. Stem Cells 27:744–752
Garcia AR, DaCosta JM, Pan J, Muenzer J, Lamsa JC (2007a) Preclinical dose ranging studies for enzyme replacement therapy with idursulfase in a knock-out mouse model of MPS II. Mol Genet Metab 91:183–190
Garcia AR, Pan J, Lamsa JC, Muenzer J (2007b) The characterization of a murine model of mucopolysaccharidosis II (Hunter syndrome). J Inherit Metab Dis 30:924–934
Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R (2009) Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years. J Pediatr 154:733–737
Hansen MD, Filipovich AH, Davies SM et al (2008) Allogeneic hematopoietic cell transplantation (HCT) in Hurler's syndrome using a reduced intensity preparative regimen. Bone Marrow Transplant 41:349–353
Higuchi T, Shimizu H, Fukuda T et al (2012) Enzyme replacement therapy (ERT) procedure for mucopolysaccharidosis type II (MPS II) by intraventricular administration (IVA) in murine MPS II. Mol Genet Metab 107:122–128
Ito K, Ochiai T, Suzuki H, Chin M, Shichino H, Mugishima H (2004) The effect of haematopoietic stem cell transplant on papules with 'pebbly' appearance in Hunter's syndrome. Br J Dermatol 151:207–211
Lessard MD, Alley TL, Proctor JL, Levy B, Galvin N, Vogler CA, Soper BW (2006) Attenuation of murine lysosomal storage disease by allogeneic neonatal bone marrow transplantation using costimulatory blockade and donor lymphocyte infusion without myeloablation. Clin Immunol 119:166–179
Link B, de Camargo Pinto LL, Giugliani R, Wraith JE, Guffon N, Eich E, Beck M (2010) Orthopedic manifestations in patients with mucopolysaccharidosis type II (Hunter syndrome) enrolled in the Hunter Outcome Survey. Orthop Rev (Pavia) 2:e16
Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA (2002) Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr Suppl 91:98–99
Muenzer J, Beck M, Eng CM et al (2009a) Multidisciplinary management of Hunter syndrome. Pediatrics 124:e1228–e1239
Muenzer J, Wraith JE, Clarke LA (2009b) International consensus panel on management and treatment of mucopolysaccharidosis I. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics 123:19–29
Muenzer J, Beck M, Giugliani R et al (2011) Idursulfase treatment of Hunter syndrome in children younger than 6 years: results from the Hunter Outcome Survey. Genet Med 13:102–109
Mullen CA, Thompson JN, Richard LA, Chan KW (2000) Unrelated umbilical cord blood transplantation in infancy for mucopolysaccharidosis type IIB (Hunter syndrome) complicated by autoimmune hemolytic anemia. Bone Marrow Transplant 25:1093–1097
Peters C, Steward CG, National Marrow Donor Program, International Bone Marrow Transplant Registry, Working Party on Inborn Errors, European Bone Marrow Transplant Group (2003) Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant 31:229–239
Prasad VK, Kurtzberg J (2008) Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant 41:99–108
Priller J, Flugel A, Wehner T et al (2001) Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med 7:1356–1361
Sato Y, Fujiwara M, Kobayashi H, Ida H (2013) Massive accumulation of glycosaminoglycans in the aortic valve of a patient with Hunter syndrome during enzyme replacement therapy. Pediatr Cardiol 34:2077–2079
Scarpa M, Almassy Z, Beck M et al (2011) Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet J Rare Dis 6:72. doi:10.1186/1750-1172-6-72
Simonaro CM, D'Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH (2008) Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am J Pathol 172:112–122
Soper BW, Lessard MD, Vogler CA, Levy B, Beamer WG, Sly WS, Barker JE (2001) Nonablative neonatal marrow transplantation attenuates functional and physical defects of beta-glucuronidase deficiency. Blood 97:1498–1504
Tanaka A, Okuyama T, Suzuki Y et al (2012) Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab 107:513–520
Vellodi A, Young E, Cooper A, Lidchi V, Winchester B, Wraith JE (1999) Long-term follow-up following bone marrow transplantation for Hunter disease. J Inherit Metab Dis 22:638–648
Wynn RF, Wraith JE, Mercer J et al (2009) Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J Pediatr 154:609–611
Yokoi T, Kobayashi H, Shimada Y et al (2011) Minimum requirement of donor cells to reduce the glycolipid storage following bone marrow transplantation in a murine model of Fabry disease. J Gene Med 13:262–268
Acknowledgments
The authors thank Joseph Muenzer of the University of North Carolina at Chapel Hill for providing the MPS II mice. We also thank Taku Sato of Tokyo Medical and Dental University for helping with the flow cytometry study. Finally, we thank the members of the Laboratory Animal Facility at The Jikei University School of Medicine for help with the animal studies. This work was supported by a KAKENHI Grant (No. 25860884) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Compliance of Ethics Guidelines
The data in the manuscript are original and the manuscript is not under consideration for publication elsewhere. None of the manuscript contents have been previously published except in abstract form.
Conflict of interest
Toya Ohashi has active research support from Genzyme Japan Co., Ltd. and Shire Japan.
Hiroyuki Ida has active research support from Genzyme Japan Co., Ltd. and Shire Japan Co., Ltd.
Kentaro Yokoi, Kazumasa Akiyama, Eiko Kaneshiro, Takashi Higuchi, Yohta Shimada, Hiroshi Kobayashi, Masaharu Akiyama, Makoto Otsu, and Hiromitsu Nakauchi declare that they have no conflict of interest.
This support has been fully disclosed and is managed under a Memorandum of Understanding with the Conflict of Interest Resolution Board of The Jikei University School of Medicine.
Contributions
K.Y., K.A. and T.O. designed and conducted research; E.K., H.H., Y.S. and H.K. performed experiments and analysed data; K.Y. wrote the paper; M.A., M.O., H.N., T.O., and H.I. discussed data and edited the paper.
Animal Rights
All intuitional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Marc Patterson
Rights and permissions
About this article
Cite this article
Yokoi, K., Akiyama, K., Kaneshiro, E. et al. Effect of donor chimerism to reduce the level of glycosaminoglycans following bone marrow transplantation in a murine model of mucopolysaccharidosis type II. J Inherit Metab Dis 38, 333–340 (2015). https://doi.org/10.1007/s10545-014-9800-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9800-x