Abstract
Introduction
Mucopolysaccharidosis type I (MPS I) results in a defective breakdown of the glycosaminoglycans (GAGs) heparan sulfate and dermatan sulfate, which leads to a progressive disease. Enzyme replacement therapy (ERT) results in clearance of these GAGs from a range of tissues and can significantly ameliorate several symptoms. The biochemical efficacy of ERT is generally assessed by the determination of the total urinary excretion of GAGs. However, this has limitations. We studied the concentrations of heparan sulfate and dermatan sulfate derived disaccharides (HS and DS, respectively) in the plasma and urine of seven patients and compared these levels with total urinary GAGs (uGAGs) levels.
Methods
Plasma and urine samples were collected at different time points relative to the weekly ERT for three non-consecutive weeks in seven MPS I patients who had been treated with ERT for at least 2.5 years. Heparan and dermatan sulfate in plasma and urine were enzymatically digested into disaccharides, and HS and DS levels were determined by HPLC-MS/MS analysis. uGAGs were measured by the DMB test.
Results
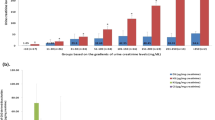
The levels of HS and DS were markedly decreased compared with the levels before the initiation of ERT. However, the concentrations of DS in plasma and of both HS and DS in urine remained significantly elevated in all studied patients, while in six patients the level of total uGAGs had normalized. The concentrations of plasma and urinary HS during the weekly ERT followed a U-shaped curve. However, the effect size is small. The concentrations of plasma and urinary DS and uGAGs appeared to be in a steady state.
Conclusions
HS and DS are sensitive biomarkers for monitoring the biochemical treatment efficacy of ERT and remain elevated despite long-term treatment. This finding may be related to the labeled dose or antibody status of the patient. The timing of the sample collection is not relevant, at least at the current dose of 100 IU/kg/weekly.


Similar content being viewed by others
References
Brooks DA, Kakavanos R, Hopwood JJ (2003) Significance of immune response to enzyme-replacement therapy for patients with a lysosomal storage disorder. Trends Mol Med 9:450–453
Church H, Tylee K, Cooper A et al (2007) Biochemical monitoring after haemopoietic stem cell transplant for Hurler syndrome (MPSIH): implications for functional outcome after transplant in metabolic disease. Bone Marrow Transplant 39:207–210
Clarke LA, Hemmelgarn H, Colobong K et al (2011) Longitudinal observations of serum heparin cofactor II-thrombin complex in treated Mucopolysaccharidosis I and II patients. J Inherit Metab Dis
Clarke LA, Wraith JE, Beck M et al (2009) Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics 123:229–240
de Jong JG, Wevers RA, Laarakkers C, Poorthuis BJ (1989) Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin Chem 35:1472–1477
de Ru MH, Boelens JJ, Das AM et al (2011) Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J Rare Dis 6:55
Giugliani R, Rojas VM, Martins AM et al (2009) A dose-optimization trial of laronidase (Aldurazyme) in patients with mucopolysaccharidosis I. Mol Genet Metab 96:13–19
Kakkis ED, Muenzer J, Tiller GE et al (2001) Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med 344:182–188
Langford-Smith K, Arasaradnam M, Wraith JE, Wynn R, Bigger BW (2010) Evaluation of heparin cofactor II-thrombin complex as a biomarker on blood spots from mucopolysaccharidosis I, IIIA and IIIB mice. Mol Genet Metab 99:269–274
Langford-Smith KJ, Mercer J, Petty J et al (2011) Heparin cofactor II-thrombin complex and dermatan sulphate:chondroitin sulphate ratio are biomarkers of short- and long-term treatment effects in mucopolysaccharide diseases. J Inherit Metab Dis 34:499–508
Lawrence R, Lu H, Rosenberg RD, Esko JD, Zhang L (2008) Disaccharide structure code for the easy representation of constituent oligosaccharides from glycosaminoglycans. Nat Methods 5:291–292
Muenzer J, Wraith JE, Clarke LA (2009) Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics 123:19–29
Oguma T, Tomatsu S, Montano AM, Okazaki O (2007) Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal Biochem 368:79–86
Pastores GM, Arn P, Beck M et al (2007) The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol Genet Metab 91:37–47
Pojasek K, Shriver Z, Kiley P, Venkataraman G, Sasisekharan R (2001) Recombinant expression, purification, and kinetic characterization of chondroitinase AC and chondroitinase B from Flavobacterium heparinum. Biochem Biophys Res Commun 286:343–351
Randall DR, Colobong KE, Hemmelgarn H et al (2008) Heparin cofactor II-thrombin complex: a biomarker of MPS disease. Mol Genet Metab 94:456–461
Randall DR, Sinclair GB, Colobong KE, Hetty E, Clarke LA (2006) Heparin cofactor II-thrombin complex in MPS I: a biomarker of MPS disease. Mol Genet Metab 88:235–243
Saif MA, Bigger BW, Brookes KE et al (2012) Hematopoietic stem cell transplantation ameliorates the high incidence of neutralizing allo-antibodies observed in MPSI-Hurler after pharmacological enzyme replacement therapy. Haematologica
Sifuentes M, Doroshow R, Hoft R et al (2007) A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab 90:171–180
Tomatsu S, Montano AM, Oguma T et al (2010a) Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J Inherit Metab Dis 33:141–150
Tomatsu S, Montano AM, Oguma T et al (2010b) Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol Genet Metab 99:124–131
Wraith JE, Beck M, Lane R et al (2007) Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human alpha-L-iduronidase (laronidase). Pediatrics 120:e37–e46
Wraith JE, Clarke LA, Beck M et al (2004) Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase). J Pediatr 144:581–588
Wynn RF, Wraith JE, Mercer J et al (2009) Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J Pediatr 154:609–611
Competing interests
FAW and CEMH received research and travel grants and the reimbursement of expenses and honoraria for lectures on lysosomal storage diseases from Genzyme Corp., USA. CEMH donated all honoraria to the Gaucher Stichting, a national foundation that supports research in the field of lysosomal storage disorders. MHdR received a travel grant from Genzyme Corp., USA. All other authors have no competing interests.
Funding
The study was partially funded by Genzyme Corp., USA. The funding source played no role in the writing of this article or in the decision to submit it for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Robin Lachmann
Minke H. de Ru and Linda van der Tol contributed equally to this study
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 3
a–g. Plasma and urinary HS and DS levels and total uGAGs relative to the day of infusion of laronidase (day 1) in different patients. HSp = HS plasma*, DSp = DS plasma**, uHS = urinary HS***, uDS = urinary DS**. Total uGAGs = total urinary GAGs as measured by the DMB test, * = represented by the disaccharide D0A0, ** = represented by the sum of the disaccharides D0a4 and D0a10, *** = represented by the sum of the disaccharides D0A0, D0A6 + D2A0, D2S0 and D0S6 + D2S0. (JPEG 533 kb)
Rights and permissions
About this article
Cite this article
de Ru, M.H., van der Tol, L., van Vlies, N. et al. Plasma and urinary levels of dermatan sulfate and heparan sulfate derived disaccharides after long-term enzyme replacement therapy (ERT) in MPS I: correlation with the timing of ERT and with total urinary excretion of glycosaminoglycans. J Inherit Metab Dis 36, 247–255 (2013). https://doi.org/10.1007/s10545-012-9538-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9538-2