Abstract
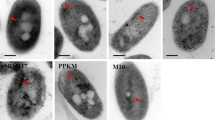
LFchimera, a construct combining two antimicrobial domains of bovine lactoferrin, lactoferrampin265–284 and lactoferricin17–30, possesses strong bactericidal activity. As yet, no experimental evidence was presented to evaluate the mechanisms of LFchimera against Burkholderia isolates. In this study we analyzed the killing activity of LFchimera on the category B pathogen Burkholderia pseudomallei in comparison to the lesser virulent Burkholderia thailandensis often used as a model for the highly virulent B. pseudomallei. Killing kinetics showed that B. thailandensis E264 was more susceptible for LFchimera than B. pseudomallei 1026b. Interestingly the bactericidal activity of LFchimera appeared highly pH dependent; B. thailandensis killing was completely abolished at and below pH 6.4. FITC-labeled LFchimera caused a rapid accumulation within 15 min in the cytoplasm of both bacterial species. Moreover, freeze-fracture electron microscopy demonstrated extreme effects on the membrane morphology of both bacterial species within 1 h of incubation, accompanied by altered membrane permeability monitored as leakage of nucleotides. These data indicate that the mechanism of action of LFchimera is similar for both species and encompasses disruption of the plasma membrane and subsequently leakage of intracellular nucleotides leading to cell dead.





Similar content being viewed by others
References
Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M (1992) Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol 73:472–479
Bolscher JG, Adao R, Nazmi K, van den Keybus PA, van ‘t Hof W, Nieuw Amerongen AV et al (2009) Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 91:123–132
Bolscher JG, Oudhoff MJ, Nazmi K, Antos JM, Guimaraes CP, Spooner E et al (2011) Sortase A as a tool for high-yield histatin cyclization. FASEB J 25:2650–2658
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250
den Hertog AL, van Marle J, Veerman EC, Valentijn-Benz M, Nazmi K, Kalay H et al (2006) The human cathelicidin peptide LL-37 and truncated variants induce segregation of lipids and proteins in the plasma membrane of Candida albicans. Biol Chem 387:1495–1502
Flores-Villasenor H, Canizalez-Roman A, Reyes-Lopez M, Nazmi K, de la Garza M, Zazueta-Beltran J et al (2010) Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 23:569–578
Flores-Villasenor H, Canizalez-Roman A, de la Garza M, Nazmi K, Bolscher JG, Leon-Sicairos N (2012) Lactoferrin and lactoferrin chimera inhibit damage caused by enteropathogenic Escherichia coli in HEp-2 cells. Biochimie 94:1935–1942
Gunn JS, Lim KB, Krueger J, Kim K, Guo L, Hackett M et al (1998) PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol 27:1171–1182
Haney EF, Lau F, Vogel HJ (2007) Solution structures and model membrane interactions of lactoferrampin, an antimicrobial peptide derived from bovine lactoferrin. Biochim Biophys Acta 1768:2355–2364
Haraga A, West TE, Brittnacher MJ, Skerrett SJ, Miller SI (2008) Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect Immun 76:5402–5411
Kanthawong S, Bolscher JG, Veerman EC, van Marle J, Nazmi K, Wongratanacheewin S et al (2010) Antimicrobial activities of LL-37 and its truncated variants against Burkholderia thailandensis. Int J Antimicrob Agents 36:447–452
Kanthawong S, Bolscher JG, Veerman EC, van Marle J, de Soet HJ, Nazmi K et al (2012) Antimicrobial and antibiofilm activity of LL-37 and its truncated variants against Burkholderia pseudomallei. Int J Antimicrob Agents 39:39–44
Kawasaki K, Ernst RK, Miller SI (2005) Inhibition of Salmonella enterica serovar Typhimurium lipopolysaccharide deacylation by aminoarabinose membrane modification. J Bacteriol 187:2448–2457
Leon-Sicairos N, Canizalez-Roman A, de la Garza M, Reyes-Lopez M, Zazueta-Beltran J, Nazmi K et al (2009) Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie 91:133–140
Limmathurotsakul D, Peacock SJ (2011) Melioidosis: a clinical overview. Br Med Bull 99:125–139
Novem V, Shui G, Wang D, Bendt AK, Sim SH, Liu Y et al (2009) Structural and biological diversity of lipopolysaccharides from Burkholderia pseudomallei and Burkholderia thailandensis. Clin Vaccine Immunol 16:1420–1428
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341(Pt 3):501–513
Puknun A, Bolscher JG, Nazmi K, Veerman EC, Tungpradabkul S, Wongratanacheewin S et al (2013) A heterodimer comprised of two bovine lactoferrin antimicrobial peptides exhibits powerful bactericidal activity against Burkholderia pseudomallei. World J Microbiol Biotechnol 29:1217–1224
Ruissen AL, Groenink J, Helmerhorst EJ, Walgreen-Weterings E, Van’t Hof W, Veerman EC et al (2001) Effects of histatin 5 and derived peptides on Candida albicans. Biochem J 356:361–368
Schweizer HP (2012) Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7:1389–1399
Silva T, Abengozar MA, Fernandez-Reyes M, Andreu D, Nazmi K, Bolscher JG et al (2012) Enhanced leishmanicidal activity of cryptopeptide chimeras from the active N1 domain of bovine lactoferrin. Amino Acids 43:2265–2277
van der Kraan MI, Groenink J, Nazmi K, Veerman EC, Bolscher JG, Nieuw Amerongen AV (2004) Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 25:177–183
van der Kraan MI, Nazmi K, Teeken A, Groenink J, van ‘t Hof W, Veerman EC et al (2005) Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol Chem 386:137–142
Wiersinga WJ, Currie BJ, Peacock SJ (2013) Melioidosis. N Engl J Med 367:1035–1044
Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ (2006) Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 4:272–282
Wuthiekanun V, Amornchai P, Saiprom N, Chantratita N, Chierakul W, Koh GC et al (2011) Survey of antimicrobial resistance in clinical Burkholderia pseudomallei isolates over two decades in Northeast Thailand. Antimicrob Agents Chemother 55:5388–5391
Acknowledgments
This work was supported by the Commission on Higher Education granting under the CHE-Ph.D.-SW and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University. JGMB, KN and ECIV are supported by a grant from the University of Amsterdam for research into the focal point Oral Infections and Inflammation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanthawong, S., Puknun, A., Bolscher, J.G.M. et al. Membrane-active mechanism of LFchimera against Burkholderia pseudomallei and Burkholderia thailandensis . Biometals 27, 949–956 (2014). https://doi.org/10.1007/s10534-014-9760-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-014-9760-5