Abstract
With global change expected to alter aspects of the carbon (C) cycle, empirical data describing how microorganisms function in different environmental conditions are needed to increase predictive capabilities of microbially-driven decomposition models. Given the importance of accelerated C fluxes during early decay in C cycling, we characterized how varying litter qualities (maple vs. oak) and sizes (ground vs. 0.25 cm2 vs. 1 cm2), and contrasting soils (sandy vs. loamy), altered microbial biomass-carbon and community structure, respiration, enzyme activities, and inorganic nutrients over the initial 2 weeks of decomposition. Our hypotheses were (1) mixing ground maple with loam should result in a quicker, more prolonged respiration response than other treatments; and (2) “priming”, or substrate-stimulated soil organic matter turnover, should be minimal over the first few days due to soluble C substrate uptake. Respiration peaks, biomass increases, nutrient immobilization, low enzyme activities, and minimal priming occurred in all treatments over the first 72 h. These general features suggest soluble C compounds are degraded before polymeric substrates regardless of litter size or type, or soil. Ground litter addition to the high C and microbial biomass loam resulted in a more prolonged respiration peak than the poorly aggregated sand. Priming was greater in loam than the C limited sandy soil after the first 72 h, likely due to co-metabolism of labile and recalcitrant substrates. We conclude that the general features of early decay are widespread and predictable, yet differences in litter and soil characteristics influence the temporal pattern and magnitude of C flux.






Similar content being viewed by others
References
Aber JD, Melillo JM, McClaugherty CA (1990) Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can J Bot 68:2201–2208
Ahn M, Zimmerman AR, Comerford MB, Sickman JO, Grunwald S (2009) Carbon mineralization and labile organic carbon pools in the sandy soils of a North Florida watershed. Ecosystems 12:672–685
Allison SD, Martiny BH (2008) Resistance, resilience, and redundancy in microbial communities. PNAS 115:11512–11519
Alvarez E, Marcos M, Torrado V, Sanjurjo M (2008) Dynamics of macronutrients during the first stages of litter decomposition from forest species in a temperate area. Nutr Cycl Agroecosyst 80:243–256
Anderson TH, Domsch KH (1989) Ratios of microbial biomass carbon to total organic-carbon in arable soils. Soil Biol Biochem 21:471–479
Anderson JM, Ineson P, Huish SA (1983) Nitrogen and cation mobilization by soil fauna feeding on leaf litter and soil organic matter from deciduous woodlands. Soil Biol Biochem 15:463–467
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133:13–22
Berg B, McClaugherty C (2008) Plant litter: decomposition, humus formation, carbon sequestration. Springer, Berlin
Bernhard-Reversat F, Main G, Hall K, Loumeto J, Ngao J (2003) Fast disappearance of the water-soluble phenolic fraction in eucalypt leaf litter during laboratory and field experiments. Appl Soil Ecol 23:273–278
Blagodatskaya EV, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y (2007) Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol 37:95–105
Bouvy M, Bettarel Y, Bouvier C, Domaizon I, Jacquet S, LeFloc’h E, Montanie H, Mostajir B, Sime-Ngando T, Torreton P, Vidussi F, Bouvier T (2011) Trophic interactions between viruses, bacteria and non flagellates under various nutrient conditions and simulated climate change. Environ Microbiol 13:1842–1857
Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE (2002) Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99:317–323
Canizares R, Benitez E, Ogunseitan OA (2011) Molecular analysis of β-glucosidase diversity and function in soil. Eur J Soil Biol 47:1–8
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst PF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
D’Angelo E, Crutchfield J, Vandiviere M (2001) Rapid, sensitive, microscale determination of phosphate in water and soil. J Environ Qual 30:2206–2209
DeForest JL, Scott LG (2010) Available organic soil phosphorus has an important influence on microbial community composition. Soil Sci Soc Am J 74:2059–2066
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition and the microbial degradation of cellobiose and vanillin in a northern hardwood forest. Soil Biol Biochem 36:965–971
DeForest JL, Smemo KA, Burke DJ, Elliott HL, Becker JC (2012) Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry 109:189–202
Doane TA, Horwath WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol 18:1781–1796
Ekschmitt K, Liu MK, Vetter S, Fox O, Wolters V (2005) Strategies used by soil biota to overcome soil organic matter stability-why is dead organic matter left over in the soil? Geoderma 128:167–176
Fierer N, Schimel JP, Holden PA (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–845
Frey SD, Drijber R, Smith H, Mellilo J (2008) Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol Biochem 40:2904–2907
Frouz J, Elhottova D, Pizl V, Tajovsky J, Sourkova M, Picek T, Maly S (2007) The effect of litter quality and soil fauna composition on organic matter dynamics in post mining soil: a laboratory study. Appl Soil Ecol 37:72–80
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Biol 6:751–765
Glanville H, Rousk J, Golyshin P, Jones DL (2012) Mineralization of low molecular weight carbon substrates in soil solution under laboratory and field conditions. Soil Biol Biochem 48:88–95
Grandy AS, Neff JC (2008) Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404:297–307
Gu L, Post W, King A (2004) Fast labile C turnover obscures sensitivity of heterotrophic respiration from soil to temperature: a model analysis. Global Biogeochem Cycl 18:1022
Guenet B, Juarez S, Bardoux G, Abbadie L, Chenu C (2012) Evidence that stable C is as vulnerable to priming effect as is more labile C in soil. Soil Biol Biochem 52:43–48
Hanlon RDG, Anderson JM (1980) Influence of macroarthropod feeding activities on macroflora in decomposing oak leaves. Soil Biol Biochem 12:255–261
Hibbard KA, Law BE, Reichstein M, Sulzman J (2005) An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry 73:29–70
Jacob M, Viedenz K, Polle A, Thomas F (2010) Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 164(4):1083–1094
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Kuzyakov Y (2005) Theoretical background for partitioning of root and rhizomicrobial respiration by δ 13C of microbial biomass. Eur J Soil Biol 41:1–9
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Bol R (2006) Sources and mechanisms of priming effect induced in two grassland soil amended with slurry and sugar. Soil Biol Biochem 38:747–758
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lambers H, Mougel C, Jaillard B, Hinsinger P (2009) Plant–microbe–soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil 321:83–115
Lovett GM, Weather KC, Arthur MA, Schultz JC (2004) Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67:289–308
McClaugherty CA, Pastor J, Aber JD, Melillo JM (1985) Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 66:266–275
Meier CL, Bowman WD (2008) Phenolic-rich leaf carbon fractions differentially influence microbial respiration and plant growth. Oecologia 158:95–107
Moni C, Rumpel C, Virto I, Chabbi A, Chenu C (2010) Relative importance of sorption versus aggregation for organic matter storage in subsoil horizons of two contrasting soils. Eur J Soil Sci 61:958–969
Moorhead DL, Reynolds JF (1993) Changing carbon chemistry of buried creosote bush litter during decomposition in the Northern Chihuahuan Desert. Am Midl Nat 130:83–89
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Moorhead DL, Lashermes G, Sinsabaugh RL (2012) A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biol Biochem 53:133–141
Mula-Michel HP, Williams MA (2012) Soil type modestly impacts bacterial community succession associated with decomposing grass detrituspheres. Soil Sci Soc Am J 77:133–144
Nielsen UN, Ayres E, Wall DH, Bardgett RD (2011) Soil biodiversity and carbon cycling: a review and synthesis of studies examining diversity–function relationships. Eur J Soil Sci 62:105–116
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49:175–190
Plante AF, Conant RT, Stewart CE, Paustian K, Six J (2006) Impact of soil texture on the distribution of soil organic matter in physical and chemical fractions. Soil Sci Soc Am J 79:287–296
Potthoff M, Steenwerth KL, Jackson LE, Drenovsky RE, Scow KM, Joergensen RG (2006) Soil microbial community composition as affected by restoration practices in California grassland. Soil Biol Biochem 38:1851–1860
Reber H, Schara A (1971) Degradation sequences in wheat straw extracts inoculated with soil suspensions. Soil Biol Biochem 3:381–383
Rhine ED, Sims GK, Mulvaney RL, Pratt EJ (1998) Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci Soc Am J 62:473–480
Rinkes ZL, Weintraub MN, DeForest JL, Moorhead DL (2011) Microbial substrate preference and community dynamics during the decomposition of Acer saccharum. Fungal Ecol 4(6):396–407
Romani AM, Fischer H, Mille-Lindblom C, Tranvik LJ (2006) Interactions of bacteria and fungi on decomposing litter: differential enzyme activities. Ecology 87:2559–2569
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sayer EJ, Powers JS, Tanner EVJ (2007) Increased litter fall in tropical forests boosts the transfer of soil CO2 to the atmosphere. PLoS One 2(12):e1299
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schlesinger WH, Andrews JA (2000) Soil respiration and the global carbon cycle. Biogeochemistry 48:7–20
Scott-Denton LE, Rosensteil TN, Monson RK (2006) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob Change Biol 12:205–216
Sexstone AJ, Revsbech NP, Parkin TB, Tiedje JM (1985) Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J 49:645–651
Sinsabaugh RL, Van Horn DJ, Follstad Shah JJ, Findlay S (2010) Ecoenzymatic stoichiometry in relation to productivity for freshwater biofilm and plankton communities. Microb Ecol 60:885–893
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–176
Snajdr J, Cajthaml T, Valaskova V, Merhautova P, Petrankova M, Spetz P, Leppanen K, Baldrian P (2011) Transformation of Quercus petraea litter: successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol Ecol 75:291–303
Snyder JD, Trofymow JA (1984) A rapid accurate wet oxidation diffusion procedure for determining organic and inorganic carbon in plants and soil. Commun Soil Sci Plant Anal 15:587–597
Steenwerth KL, Jackson LE, Carlisle EA, Scow KM (2006) Microbial communities of a native perennial bunchgrass do not respond consistently across a gradient of land-use intensification. Soil Biol Biochem 38:1797–1811
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 93(2):345–354
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Treseder KK, Balser TC, Bradford MA, Brodie EL, Dubinsky EA, Eviner VT, Hofmockel KS, Lennon JT, Levine UY, MacGregor BJ, Pett-Ridge J, Waldrop MP (2011) Integrating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry 109:7–18
Van Hees PAW, Jones DL, Finlay R, Godbold DL, Lundstrom US (2005) The carbon we do not see-the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biol Biochem 37:1–13
Waldrop MP, Firestone MK (2004) Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138:275–284
Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling the in situ activity of extracellular enzymes. Soil Biol Biochem 40:2098–2106
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51–62
Wickings K, Grandy AS (2011) The oribatid mite Scheloribates moestus (Acari: Oribitada) alters litter chemistry and nutrient cycling during decomposition. Soil Biol Biochem 43:351–358
Wickings K, Grandy AS, Reed S, Cleveland C (2011) Management intensity alters decomposition via biological pathways. Biogeochemistry 104:365–379
Wickings K, Grandy AS, Reed S, Cleveland C (2012) The origin of litter chemical complexity during decomposition. Ecol Lett 15:1180–1188
Wolters V (2000) Invertebrate control of soil organic stability. Bio Fertil Soils 31:1–19
Xin WD, Yin XQ, Song B (2012) Contribution of soil fauna to litter decomposition in Songnen sandy lands in northeastern China. J Arid Environ 77:90–95
Yang X, Yang Z, Warren MW, Chen J (2012) Mechanical fragmentation enhances the contribution of Collembola to leaf litter decomposition. Eur J Soil Biol 53:23–31
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Acknowledgments
This research was supported by the NSF Ecosystems Program (Grant # 0918718). For field and laboratory assistance, we thank Mallory Ladd, Ryan Monnin, Steve Solomon, Heather Thoman, Logan Thornsberry, and Megan Wenzel. We are also grateful to Jason Witter for assistance in freeze-drying samples for PLFA analysis. For help with CN analysis, we thank Doug Sturtz, Russ Friederich, and Jonathan Frantz from the USDA ARS at the University of Toledo. We also thank two anonymous reviewers whose suggestions greatly improved this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Colin Bell
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
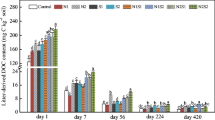
Dissolved organic carbon (DOC) concentrations over time during the two-week incubation for A sugar maple and B oak treatments. Values are expressed as μg-C g dry soil−1. Error bars show the standard error of the mean (n = 4). Tukey’s posthoc test was used to determine significant differences between treatments. Lowercase letters were used to designate significant differences between treatments. (TIFF 231 kb)
Rights and permissions
About this article
Cite this article
Rinkes, Z.L., DeForest, J.L., Grandy, A.S. et al. Interactions between leaf litter quality, particle size, and microbial community during the earliest stage of decay. Biogeochemistry 117, 153–168 (2014). https://doi.org/10.1007/s10533-013-9872-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9872-y