Abstract
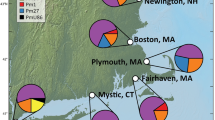
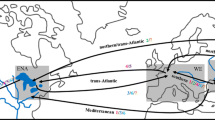
Genetic variation can be used to determine routes of introduction of non-native species and whether introduced populations lost variation during establishment. The present study sought to determine whether multiple, geographically isolated non-native populations of the green mussel, Perna viridis, were the product of a stepping stone expansion of a single introduction or from multiple independent introductions from the native range. Measurements of genetic variation were compared among five introduced populations and three populations from within the native range. We sequenced 650 bp of the mitochondrial gene cytochrome oxidase I from 280 samples from five introduced populations and another 190 samples from three native populations. Haplotype frequencies of all introduced populations were not significantly different from each other, but virtually all populations differed from samples taken from the native range. Measurements of genetic variation tended to suggest that introduced populations had less variation than most native populations and there was no evidence for admixture in any of the introduced populations. The genetic data and Monte Carlo simulations both provide compelling evidence of a stepping-stone pattern of introduction of P. viridis from the native range to Trinidad, and from Trinidad to other locations in the Caribbean and United States. The lack of genetic variation in introduced populations suggests that the initial introduction was relatively small and the lack of admixture suggests a single original source population.






Similar content being viewed by others
References
Agard J, Kishore R, Bayne B (1992) Perna viridis (Linnaeus, 1758): first record of the Indo-Pacific green mussel (Mollusca: Bivalvia) in the Caribbean. Caribb Mar Stud 3:59–60
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conserv Biol 17:24–30
Astanei I, Gosling E, Wilson J, Powell E (2005) Genetic variability and phylogeography of the invasive zebra mussel, Dreissena polymorpha (Pallas). Mol Ecol 14:1655–1666
Baker P, Benson A (2002) Habitat and ecology of green mussels, Perna viridis, in Florida. J Shellfish Res 21:424–425
Baker AJ, Moeed A (1987) Rapid genetic differentiation and founder effect in colonizing populations of common mynas (Acridotheres tristas). Evolution 41:525–538
Barber BJ, Fajans JS, Baker SM, Baker P (2005) Gametogenesis in the non-native green mussel, Perna viridis, and the native scorched mussel, Brachidonte sexustus, in Tampa Bay, Florida. J Shell Res 24:1087–1095
Barbiero RP, Rockwell DC, Warren GJ, Tuchman ML (2006) Changes in spring phytoplankton communities and nutrient dynamics in the eastern basin of Lake Erie since the invasion of Dreissena spp. Can J Fish Aquat Sci 63:1549–1563
Baskin Y (1998) Winners and losers in a changing world: global changes may promote invasions and alter the fate of invasive species. Bioscience 48:788–792
Benson JJ, Marelli DC, Frischer ME, Danforth JM, Williams JD (2001) Establishment of the green mussel Perna viridis (Linnaeus 1758), (Mollusca: Mytilidae) on the west coast of Florida. J Shellfish Res 20:21–29
Bohonak AJ, Davies N, Villablanca FX, Roderick GC (2001) Invasion genetics of New World medflies: testing alternative colonization scenarios. Biol Invasions 3:103–111
Buddo DSTA, Steele RD, D’Oyen ER (2003) Distribution of the invasive Indo-Pacific green mussel, Perna viridis, in Kingston Harbour, Jamaica. Bull Mar Sci 73:433–441
Carlton JT (1985) Transoceanic and interoceanic dispersal of coastal marine organisms—the biology of ballast water. Oceanogr Mar Biol 23:313–371
Carlton JT (1993) Neoextinctions of marine invertebrates. Am Zool 33:499–509
Carlton JT (1996) Biological invasions and cryptogenic species. Ecology 77:1653–1655
Carlton JT, Geller JB (1993) Ecological roulette—the global transport of nonindigenous marine organisms. Science 261:78–82
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659
Colautti RI, Bailey SA, van Overdijk CDA, Amundsen K, MacIsaac HJ (2006) Characterised and projected costs of nonindigenous species in Canada. Biol Invasion 8:45–59
Dobiesz NE, McLeish DA, Eshenroder RL, Bence JR, Mohr LC, Ebener MP, Nalepa TF, Woldt AP, Johnson JE, Argyle RL, Makarewicz JC (2005) Ecology of the Lake Huron fish community, 1970–1999. Can J Fish Aquat Sci 62:1432–1451
Drake JM, Brossenbroek JM (2004) The potential distribution of zebra mussels in the United States. Bioscience 54:931–941
Excoffier L, Smouse P, Quattro J (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evolut Bioinform Online 1:47–50
Firth LB, Knights AM, Bell SS (2011) Air temperature and winter mortality: implications for the persistence of the invasive mussel, Perna viridis in the intertidal zone of the south-eastern United States. J Exp Mar Biol Ecol 400:250–256
Frankham R, Ralls K (1998) Conservation biology—inbreeding leads to extinction. Nature 392:441–442
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Fu Y-X (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Geller JB, Darling JA, Carlton JT (2010) Genetic perspectives on marine biological invasions. Ann Rev Mar Sci 2:367–393
Gillis NK, Walters LJ, Fernandes FC, Hoffman EA (2009) Higher genetic diversity in introduced than in native populations of the mussel Mytellacharruana: evidence of population admixture at introduction sites. Diversity Distrib 15:784–795
Grant WS, Cherry MI (1985) MytilusgalloprovincialisLmk. In southern Africa. J Exp Mar Biol Ecol 90:179–191
Griffiths CL, Hockey PAR, Schurink CV, Leroux PJ (1992) Marine invasive aliens on South African shores—implications for community structure and trophic functioning. Afrikaanse Tydskrif Vir Wetenskap 12:713–722
Harpending HC, Sherry ST, Stoneking M, Rogers AR (1993) The genetic structure of ancient human populations. Curr Anthropol 34:483–496
Holland BS (2001) Invasion without a bottleneck: microsatellite variation in natural and invasive populations of the brown mussel Pernaperna (L). Mar Biotech 3:407–415
Ingrao DA, Mikkelson PM, Hicks DW (2001) Another introduced marine mollusk in the Gulf of Mexico: the Indo-Pacific green mussel, Perna viridis, in Tampa Bay, Florida. J Shellfish Res 20:13–19
Kelly DW, Muirhead JR, Heath DD, MacIssac HJ (2006) Contrasting patterns in genetic diversity following multiple invasions of fresh and brackish waters. Mol Ecol 15:3641–3653
Kolbe JJ, Glor RE, Shettino LR, Lara AC, Larson A, Losos JB (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181
Lammens E, Van Nes EH, Mooij WM (2002) Differences in the exploitation of bream in three shallow lake systems and their relation to water quality. Fresh Biol 47:2435–2442
Le Roux JJ, Wieczorek AM (2009) Molecular systematic and population genetics of biological invasions: towards a better understanding of invasive species management. Ann Appl Biol 154:1–17
Lockwood JL, Hoops MF, Marchetti MP (2007) Invasion ecology. Blackwell Publishing, Malden
MacArthur RH, Wilson EO (1968) The theory of island biogeography. Princeton University Press, Princeton
Marsden JE, Spidle AP, May B (1996) Review of genetic studies of Dreissena spp. Am Zool 36:259–270
May GE, GelembiukGW PanovVE, Orlova MI, Lee CE (2006) Molecular ecology of zebra mussel invasions. Mol Ecol 15:1021–1031
McCabe DJ, Beekey MA, Mazloff A, Marsden JE (2006) Negative effect of zebra mussels on foraging and habitat use by lake sturgeon (Acipenserfulvescens). Aquat Cons Mar Fresh Ecos 16:493–500
Muirhead JR, Gray DK, Kelly DW, Ellis SM, Heath DD, MacIsaac HJ (2008) Identifying the source of species invasions: sampling intensity vs. genetic diversity. Mol Ecol 17:1020–1035
Nalepa TF (1994) Decline of native unionid bivalves in Lake St. Clair after infestation by the zebra mussel. Dreissenapolymorpha. Can J Fish Aquat Sci 51:2227–2233
Novak SJ, Mack RN (2005) Genetic bottlenecks in alien plant species: influence of mating system and introduction dynamics. In: Sax DF, Gaines SD, Stachowicz JJ (eds) Exotic species—bane to conservation and boon to understanding: ecology, evolution and biogeography. Sinauer, MA, pp 95–122
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of noninigenous species in the United States. Bioscience 50:53–65
Power AJ, Walker RL, Payne K, Hurley D (2004) First occurrence of the nonindigenous green mussel, Perna viridis in coastal Georgia, United States. J Shellfish Res 23:741–744
Rajagopal S, Venugopalan VP, van der Velde G, Jenner HA (2006) Greening of the coasts: a review of the Perna viridis success story. Aquat Ecol 40:273–297
Roberts L (1990) Zebra mussel invasion threatens United States waters. Science 249:1370–1372
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22:454–464
Ross KG, Vargo EL, Keller L, Trager JC (1993) Effect of a founder event on variation on the genetic sex-determining system of the fire ant Solenopsisinvicta. Genetics 135:843–854
Rozas J, Rozas R (1999) DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174–175
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Rylander K, Perez J, Gomez JA (1996) Status of the green mussel, Perna viridis (Linnaeus, 1758)(Mollusca: Mytilidae), in northeastern Venezuela. Caribb Mar Stud 5:86–87
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmitesaustralis, into North America. Proc Nat Acad Sci USA 99:2445–2449
Schloesser DW, Nalepa TF (1994) Dramatic decline of unionid bivalves in offshore waters of western Lake Erie after infestation by the zebra mussel, Dreissenapolymorpha. Can J Fish Aquat Sci 51:2234–2242
Sebastian CR, Steffani CN, Branch GM (2002) Homing and movement patterns of a South African limpet Scutellastraargenvillei in an area invaded by an alien mussel Mytilusgallooprovincialis. Mar Ecol Prog Ser 243:111–122
Staton SK, Metcalfe-Smith JL, West EL (2000) Status of the Northern Riffleshell, Epioblasmatorulosarangiana (Bivalvia: Unionidae), in Ontario and Canada. Can Field Nat 114:224–235
Stepien CA, Taylor CD, Dabrowska KA (2002) Genetic variability and phylogeographical patterns of a nonindigenous species invasion: a comparison of exotic vs. native zebra and quagga mussel populations. J Evol Biol 15:314–328
Stepien CA, Brown JE, Neilson ME, Tumeo MA (2005) Genetic diversity of invasive species in the Great Lakes versus their Eurasian source populations: insights for risk analysis. Risk Anal 25:1043–1060
Suarez AV, Tsutsui ND, Holway DA, Case TM (1999) Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol Invasion 1:43–53
Tajima F (1989a) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tajima F (1989b) The effect of change in population size on DNA polymorphism. Genetics 123:597–601
Taylor DR, Keller SR (2007) Historical range expansion determines the phylogenetic diversity introduced during contemporary species invasion. Evolution 61:334–345
Templeton AR, Crandall KA, Sing CF (1992) Acladistic-analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA-sequence data. 3 cladogram estimation. Genetics 132:619–633
Urian AG, Hatle JH, Gilg MR (2011) Thermal constraints for range expansion of the invasive green mussel, Perna viridis, in the Southeastern United States. J Exp Zoo Part A 315A:12–21
Therriault TW, Orolova MI, Docker MF, MacIsaac HJ, Heath DD (2005) Invasion genetics of a freshwater mussel (Dreissenarostriformisbugensis) in eastern Europe: high gene flow and multiple introductions. Heredity 95:16–23
Villesen P (2007) FaBox: an online toolbox for fasta sequences. Mol Ecol Notes 7:965–968
Wares JP, Hughes AR, Grosberg RK (2005) Mechanisms that drive evolutionary change: insights from species introductions and invasions. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions, insights into ecology, evolution, and biogeography. Sinauer and Associates, Sunderland, pp 229–257
Williamson M (1996) Biological invasions. Chapman and Hall, London
Acknowledgments
The authors would like to thank Ryan Howard, Angel Beebe, Isaac Lam, the Marine Biology department at Cochin University of Science and Technology, and Camilo Trench (University of the West Indies, Mona) for their help with collecting mussels for use in this study. Also, thanks to Matthew Farrer and his research technicians for assistance and the use of their DNA sequencing equipment. The authors would also like to thank E.A. Hoffman for reviewing earlier versions of this manuscript. This project was supported through funding from USDA National Institute of Food and Agriculture award no. 2008-32320-04574 and the University of North Florida Transformational Learning Opportunities and Coastal Biology programs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilg, M.R., Johnson, E.G., Gobin, J. et al. Population genetics of introduced and native populations of the green mussel, Perna viridis: determining patterns of introduction. Biol Invasions 15, 459–472 (2013). https://doi.org/10.1007/s10530-012-0301-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0301-2