Abstract
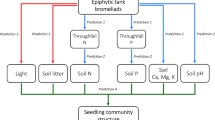
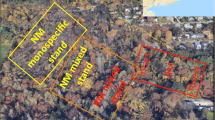
Since several decades, American boxelder (Acer negundo) is replacing white willow (Salix alba) riparian forests along southern European rivers. This study aims to evaluate the consequences of boxelder invasion on understory community in riparian areas. We determined the understory species richness, composition and biomass in boxelder and white willow stands located in three riparian forests, representative of three rivers with distinct hydrological regimes. We investigated correlation of these variables to soil moisture and particle size, main soil nutrient stocks, potential nitrification and denitrification, tree canopy cover and photosynthetic active radiation (PAR) at the ground level. A greenhouse experiment was then conducted to identify the causal factors responsible for changes in the understory. The effect of soil type, PAR level and water level on the growth and the biomass production of Urtica dioica were examined. A lower plant species richness and biomass, and a modification of community composition were observed for boxelder understory in all sites, regardless of their environmental characteristics. The strongest modification that follows boxelder invasion was the decline in U. dioica, the dominant species of the white willow forest understory. These differences were mainly correlated with a lower incident PAR under boxelder canopy. The greenhouse experiment identified PAR level as the main factor responsible for the changes in U. dioica stem number and biomass. Our results indicate that adult boxelder acts as an ecosystem engineer that decreases light availability. The opportunistic invasion by boxelder leads to important understory changes, which could alter riparian ecosystem functioning.




Similar content being viewed by others
References
Abelleira Martinez OJ (2010) Invasion by native tree species prevents biotic homogenization in novel forests of Puerto Rico. Plant Ecol 211:49–64
Aguiar FC, Ferreira MT (2005) Human-disturbed landscapes: effects on composition and integrity of riparian woody vegetation in the Tagus River basin, Portugal. Environ Conserv 32:30–41
Barsoum N (2002) Relative contributions of sexual and asexual regeneration strategies in Populus nigra and Salix alba during the first years of establishment on a braided gravel bed river. Evol Ecol 15:255–279
Bendix J, Hupp CR (2000) Hydrological and geomorphological impacts on riparian plant communities. Hydrol Process 14:2977–2990
Bottollier-Curtet M, Charcosset JY, Planty-Tabacchi AM, Tabacchi E (2011) Degradation of native and exotic riparian plant leaf litter in a floodplain pond. Freshw Biol 56:1798–1810
Braun-Blanquet J (1932) Plant sociology, the study of plant community. McGraw Hill Book, New York
Canham CD, Burbank DH (1994) Causes and consequences of resource heterogeneity in forests: interspecific variation in light transmission by canopy trees. Can J For Res 24:337–349
Chabrerie O, Loinard J, Perrin S, Saguez R, Decocq G (2010) Impact of Prunus serotina invasion on understory functional diversity in a European temperate forest. Biol Invasions 12:1891–1907
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
DAISIE (2011) European invasive alien species gateway. Retrieved from http://www.europe-aliens.org
Dassonville N, Guillaumaud N, Piola F, Meerts P, Poly F (2011) The niche construction by the invasive Asian knotweeds (species complex Fallopia): a microbial point of view. Biol Invasions 13:1115–1133
Day RL, Laland KN, Odling-Smee J (2003) Rethinking adaptation—the niche-construction perspective. Perspect Biol Med 46:80–95
Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20:470–474
Doncaster CP, Davey AJH (2010) Analysis of variance and covariance: how to choose and construct models for the life sciences. Cambridge University Press, Cambridge
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Duval L (1963) Etude des conditions de validité du dosage céruléomolybdique de l’acide phosphorique. Conséquences pratiques. Chime Anal 45:237–250
Ehrenfeld JG, Kourtev K, Huang W (2001) Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11:1287–1300
Flory SL, Clay K (2010) Non-native grass invasion suppresses forest succession. Oecologia 164:1029–1038
Friedman JM, Auble GT (1999) Mortality of riparian boxelder from sediment mobilization and extended inundation. Regul River 15:463–476
Galbraith-Kent SL, Handel SN (2008) Invasive Acer platanoides inhibits native sapling growth in forest understorey communities. J Ecol 96:293–302
Gilliam FS, Roberts MR (2003) Interactions between the herbaceous layer and overstory canopy of eastern forests: a mechanism for linkage. In: Giliam FS, Roberts MR (eds) The herbaceous layer in forests of eastern north america. Oxford University Press, New-York, pp 198–223
Hejda M, Pysek P (2006) What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol Conserv 132:143–152
Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater PB, Cramer VA, Epstein RP, Ewel JJ, Klink CA, Lugo AE, Norton D, Ojima D, Richardson DM, Sanderson EW, Valladares F, Vilà M, Zamora R, Zobel M (2006) Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob Ecol Biogeogr 15:1–7
Hoffmann WA, Haridasan M (2008) The invasive grass, Melinis minutiflora, inhibits tree regeneration in a Neotropical savanna. Austral Ecol 33:29–36
Hood WG, Naiman RJ (2000) Vulnerability of riparian zones to invasion by exotic vascular plants. Plant Ecol 148:105–114
House JI, Archer S, Breshears DD, Scholes RJ, NCEAS (2003) Conundrums in mixed woody–herbaceous plant systems. J Biogeogr 30:1763–1777
Hrázský Z (2005) Acer nagundo L. in the Czech Republic: invaded habitats and potential distribution modelling. Master Thesis, University of South Bohemia, České Budějovice, p 48
James M (1996) Le dépérissement des boisements riverains de la Garonne: évaluation à partir de données de structure forestière et de télédétection à haute résolution spatiale. PhD Thesis, University Toulouse III, Toulouse, p 196
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Klimešová J (1994) The effects of timing and duration of floods on growth of young plants of Phalaris arundinacea L. and Urtica dioica L.—an experimental study. Aquat Bot 48:21–29
Krivanek M, Pysek P, Jarosik V (2006) Planting history and propagule pressure as predictors of invasion by woody species in a temperate region. Conserv Biol 20:1487–1498
Lambdon PW, Lloret F, Hulme PE (2008) Do non-native species invasions lead to biotic homogenization at small scales? The similarity and functional diversity of habitats compared for alien and native components of Mediterranean floras. Divers Distrib 14:774–785
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc Biol Sci 270:775–781
Litton CM, Sandquist DR, Cordell S (2006) Effects of non-native grass invasion on aboveground carbon pools and tree population structure in a tropical dry forest of Hawaii. For Ecol Manag 231:105–113
Maeglin RR, Ohmann LF (1973) Boxelder (Acer negundo): a review and commentary. Bull Torrey Bot Club 100:357–363
Maule HG, Andrews M, Morton JD, Jones AV, Daly GT (1995) Sun/shade acclimatation and nitrogen nutrition of Tradescantia fluminensis, a problem weed in New Zealand native forest remnant. N Z J Ecol 19:35–46
Meekins JF, McCarthy BC (2001) Effect of environmental variation on the invasive success of a non indigenous forest herb. Ecol Appl 11:1336–1348
Mortenson SG, Weisberg PJ (2010) Does river regulation increase the dominance of invasive woody species in riparian landscapes? Glob Ecol Biogeogr 19:562–574
Mosner E, Schneider S, Lehmann B, Leyer I (2011) Hydrological prerequisites for optimum habitats of riparian Salix communities—identifying suitable reforestation sites. Appl Veg Sci 14:367–377
NFISO11263 (1995) Qualité du sol—Dosage du phosphore, Paris, p 6
Olsen C (1921) The ecology of Urtica dioica. J Ecol 9:1–18
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular of United States Department of Agriculture 939: 1–19
Orr SP, Rudgers JA, Clay K (2005) Invasive plants can inhibit native tree seedlings: testing potential allelopathic mechanisms. Plant Ecol 181:153–165
Patra KA, Abbadie L, Clays-Josserand A, Degrange V, Grayston SJ, Loiseau P, Louault F, Mahmood S, Nazaret S, Philippot L, Poly F, Prosser JI, Richaume A, Le Roux X (2005) Effect of grazing on microbial functional groups involved in soil N dynamics. Ecol Monogr 75:65–80
Planty-Tabacchi AM, Tabacchi E, Naiman RJ, Deferrari C, Decamps H (1996) Invasibility of species-rich communities in riparian zones. Conserv Biol 10:598–607
Pyšek P, Richardson DM (2010) Invasive species, environmental change and management, and health. Annu Rev Environ Resour 35:25–55
Reinhart KO, Greene E, Callaway RM (2005) Effects of Acer platanoides invasion on understory plant communities and tree regeneration in the northern Rocky Mountains. Ecography 28:573–582
Reinhart KO, Gurnee J, Tirado R, Callaway RM (2006) Invasion through quantitative effects: intense shade drives native decline and invasive success. Ecol Appl 16:1821–1831
Richardson DM, Holmes PM, Esler KJ, Galatowitsch SM, Stromberg JC, Kirkman SP, Pysek P, Hobbs RJ (2007) Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Divers Distrib 13:126–139
Saccone P, Brun JJ, Michalet R (2010a) Challenging growth-survival trade-off: a key for Acer negundo invasion in European floodplain. Can J For Res 40:1879–1886
Saccone P, Pagès JP, Girel J, Brun JJ, Michalet R (2010b) Acer negundo invasion along a successional gradient: early direct facilitation by native pioneers and late indirect facilitation by conspecifics. New Phytol 187:831–842
Sankey TT (2007) Woody-herbaceous-livestock species interaction. Ann Arid Zone 46:1–28
Searle PL (1984) The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. Analyst 109:549–568
Seastedt TR, Hobbs RJ, Suding KN (2008) Management of novel ecosystems: are novel approaches required? Front Ecol Environ 6:547–553
Siemann E, Rogers WE (2003) Changes in light and nitrogen availability under pioneer trees may indirectly facilitate tree invasions of grasslands. J Ecol 91:923–931
Siemens TJ, Blossey B (2007) An evaluation of mechanisms preventing growth and survival of two native species in invasive bohemian knotweed (Fallopia x bohemica, Polygonaceae). Am J Bot 94:776–783
Smith MS, Tiedje JM (1979) Phases of denitrification following oxygen depletion in soil. Soil Biol Biochem 11:262–267
Šrůtek M (1997) Growth responses of Urtica dioica L. to different water table depth. Plant Ecol 130:163–169
Stavisky A, Golay MJE (1964) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36:1627–1639
Steiger J, James M, Gazelle F (1998) Channelization and consequences on floodplain system functioning on the Garonne River, SW France. Regul River 14:13–23
Stohlgren TJ, Bull KA, Otsuki Y, Villa CA, Lee M (1998) Riparian zones as havens for exotic plant species in the central grasslands. Plant Ecol 138:113–125
Strickland MS, Devore JL, Maerz JC, Bradford MA (2010) Grass invasion of a hardwood forest is associated with declines in belowground carbon pools. Glob Chang Biol 16:1338–1350
Stromberg JC, Lite SJ, Marler R, Paradzick C, Shafroth PB, Shorrock D, White JM, White MS (2007) Altered stream-flow regimes and invasive plant species: the Tamarix case. Glob Ecol Biogeogr 16:381–393
Tabacchi E, Planty-Tabacchi AM (2003) Recent changes in riparian vegetation: possible consequences on dead wood processing along rivers. River Res Appl 19:251–263
Tabacchi E, Planty-Tabacchi AM (2005) Exotic and native plant community distributions within complex riparian landscapes: a positive correlation. Ecoscience 12:412–423
Taylor K (2009) Biological Flora of the British Isles: Urtica dioica L. J Ecol 97:1436–1458
Tickner DP, Angold PG, Gurnell AM, Mountford JO (2001) Riparian plant invasions: hydrogeomorphological control and ecological impacts. Prog Phys Geog 25:22–52
Torossian C, Roques L (1989) Cycle biologique et importance appliquée de l’espèce Yponomeuta rorellus Hubner dans les ripisylves à Salix alba de la région Midi Pyrénées. Acta Oecol-Oec Appl 10:47–63
Vilà M, Sardans J (1999) Plant competition in mediterranean-type vegetation. J Veg Sci 10:281–294
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošik V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Acknowledgments
We thank N. Guillaumaud for her technical assistance with measuring nitrifying and denitrifying activity, J. Sylvestre for his help for the greenhouse experiment and J. Moro for making the field access easier. The comments and suggestions of the reviewers improved the manuscript. We thank Dr. B. Loveall for English proofreading of the manuscript. M. Bottollier-Curtet was supported by a French MESR fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bottollier-Curtet, M., Charcosset, JY., Poly, F. et al. Light interception principally drives the understory response to boxelder invasion in riparian forests. Biol Invasions 14, 1445–1458 (2012). https://doi.org/10.1007/s10530-011-0170-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0170-0