Abstract
Objectives
To design novel 3D in vitro co-culture models based on the RGD-peptide-induced cell self-assembly technique.
Results
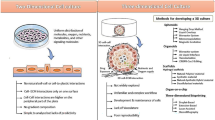
Multicellular spheroids from M-3 murine melanoma cells and L-929 murine fibroblasts were obtained directly from monolayer culture by addition of culture medium containing cyclic RGD-peptide. To reach reproducible architecture of co-culture spheroids, two novel 3D in vitro models with well pronounced core–shell structure from tumor spheroids and single mouse fibroblasts were developed based on this approach. The first was a combination of a RGD-peptide platform with the liquid overlay technique with further co-cultivation for 1–2 days. The second allowed co-culture spheroids to generate within polyelectrolyte microcapsules by cultivation for 2 weeks. M-3 cells (a core) and L-929 fibroblasts (a shell) were easily distinguished by confocal microscopy due to cell staining with DiO and DiI dyes, respectively.
Conclusions
The 3D co-culture spheroids are proposed as a tool in tumor biology to study cell–cell interactions as well as for testing novel anticancer drugs and drug delivery vehicles.





Similar content being viewed by others
References
Akasov R, Borodina T, Zaytseva E et al (2015) Ultrasonically assisted polysaccharide microcontainers for delivery of lipophilic antitumor drugs: preparation and in vitro evaluation. ACS Appl Mater Interface 7:16581–16589
Akasov R, Zaytseva-Zotova D, Burov S et al (2016) Formation of multicellular tumor spheroids induced by cyclic RGD-peptides and use for anticancer drug testing in vitro. Int J Pharm 506:148–157
Bartkowiak A, Brylak W (2006) Hydrogel microcapsules containing natural and chemically modified oligochitosans: mechanical properties and porosity. Polimery/Polymers 51:547–554
Brandner JM, Haass NK (2013) Melanoma’s connections to the tumour microenvironment. Pathology 45:443–452
Correia CR, Pirraco RP, Cerqueira MT et al (2016) Semipermeable capsules wrapping a multifunctional and self-regulated co-culture microenvironment for osteogenic differentiation. Sci Rep 6:21883
Costa EC, Gaspar VM, Coutinho P, Correia IJ (2014) Optimization of liquid overlay technique to formulate heterogenic 3D co-cultures models. Biotechnol Bioeng 111:1672–1685
Dittmer J, Leyh B (2015) The impact of tumor stroma on drug response in breast cancer. Semin Cancer Biol 31:3–15
Estrada MF, Rebelo SP, Davies EJ et al (2016) Modelling the tumour microenvironment in long-term microencapsulated 3D co-cultures recapitulates phenotypic features of disease progression. Biomaterials 78:50–61
Fang X, Sittadjody S, Gyabaah K et al (2013) Novel 3D co-culture model for epithelial-stromal cells interaction in prostate cancer. PLoS One 8:e75187
Flach EH, Rebecca VW, Herlyn M et al (2011) Fibroblasts contribute to melanoma tumor growth and drug resistance. Mol Pharm 8:2039–2049
Hickman J, Graeser R, de Hoogt R et al (2014) Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnol J 9:1115–1128
Horie M, Saito A, Yamaguchi Y et al (2015) Three-dimensional co-culture model for tumor–stromal interaction. J Vis Exp 96:e52469
Hsiao AY, Tung Y-C, Qu X et al (2012) 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechnol Bioeng 109:1293–1304
Ivanov DP, Parker TL, Walker DA et al (2015) In vitro co-culture model of medulloblastoma and human neural stem cells for drug delivery assessment. J Biotechnol 205:3–13
Jones TS, Holland EC (2012) Standard of care therapy for malignant glioma and its effect on tumor and stromal cells. Oncogene 31:1995–2006
Mehta G, Hsiao AY, Ingram M et al (2012) Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 164:192–204
Metzger W, Sossong D, Bächle A et al (2011) The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 13:1000–1012
No DY, Lee S-A, Choi YY et al (2012) Functional 3D human primary hepatocyte spheroids made by co-culturing hepatocytes from partial hepatectomy specimens and human adipose-derived stem cells. PLoS One 7:e50723
Sutherland RM, McCredie JA, Inch WR (1971) Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst 46:113–120
Zaytseva-Zotova D, Balysheva V, Tsoy A et al (2011a) Biocompatible smart microcapsules based on chitosan-poly(vinyl alcohol) copolymers for cultivation of animal cells. Adv Eng Mater 13:493–500
Zaytseva-Zotova DS, Udartseva OO, Andreeva ER et al (2011b) Polyelectrolyte microcapsules with entrapped multicellular tumor spheroids as a novel tool to study the effects of photodynamic therapy. J Biomed Mater Res 97B:255–262
Supporting Information
Supplementary Fig. 1—Spheroid size distributions and micrographs of spheroids generated from M-3 and L-929 cells at various cell count ratios.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2016_2218_MOESM1_ESM.tif
Supplementary Fig. 1. Spheroid size distributions and micrographs of spheroids generated from M-3 and L-929 cells at various cell count ratios. To form spheroids, the cells were incubated with 50 µM of cyclo-RGDfK(TPP) for 72 h. Scale bar 100 µm. (TIFF 6955 kb)
Rights and permissions
About this article
Cite this article
Akasov, R., Gileva, A., Zaytseva-Zotova, D. et al. 3D in vitro co-culture models based on normal cells and tumor spheroids formed by cyclic RGD-peptide induced cell self-assembly. Biotechnol Lett 39, 45–53 (2017). https://doi.org/10.1007/s10529-016-2218-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2218-9