Abstract
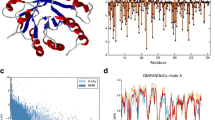
We constructed a library of chimeras from the major endoglucanase, CelA, of Clostridium thermocellum and a less stable endoglucanase CelB from Clostridium josui with multiple point mutations using low-fidelity family-shuffling method. Mutations that inactivated the enzyme were rapidly eliminated with high-throughput screening. The activities and thermostabilities of selected variants were evaluated, and four amino acid substitutions, K249R, P258S, S329N and E355G, were identified as having significant impact on the thermostability of CelA without affecting enzymatic activity. In the crystal structure of CelA, most of them are away from the activity cleft and are responsible for the stabilization of secondary structures.




Similar content being viewed by others
References
Abecassis V, Pompon D, Truan G (2000) High efficiency family shuffling based on multi-step PCR and in vivo DNA recombination in yeast: statistical and functional analysis of a combinatorial library between human cytochrome P450 1A1 and 1A2. Nucleic Acids Res 28:e88
Aiba S, Kitai K, Imanaka T (1983) Cloning and expression of thermostable α-amylase gene from Bacillus stearothermophilus in Bacillus stearothermophilus and Bacillus subtilis. Appl Environ Microbiol 46:1059–1065
Alzari PM, Souchon H, Dominguez R (1996) The crystal structure of endoglucanase CelA, a family 8 glycosyl hydrolase from Clostridium thermocellum. Structure 4:265–275
Chakravarty S, Varadarajan R (2000) Elucidation of determinants of protein stability through genome sequence analysis. FEBS Lett 470:65–69
Chakravarty S, Varadarajan R (2002) Elucidation of factors responsible for enhanced thermal stability of proteins: a structural genomics based study. Biochemistry 41:8152–8161
Fujino T, Ohmiya K (1991) Cloning of the CelB gene encoding endo-1,4-beta-glucanase-2 from Clostridium josui in Escherichia coli and the properties of the translated product. J Ferment Bioeng 72:422–425
Gold ND, Martin VJ (2007) Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J Bacteriol 189:6787–6795
Guerin DM, Lascombe MB, Costabel M, Souchon H, Lamzin V, Beguin P, Alzari PM (2002) Atomic (0.94 A) resolution structure of an inverting glycosidase in complex with substrate. J Mol Biol 316:1061–1069
Guruprasad K, Rajkumar S (2000) Beta- and gamma-turns in proteins revisited: a new set of amino acid turn-type dependent positional preferences and potentials. J Biosci (Bangalore) 25:143–156
Hoover DM, Lubkowski J (2002) DNAWorks: an automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res 30:e43
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Melchionna S, Sinibaldi R, Briganti G (2006) Explanation of the stability of thermophilic proteins based on unique micromorphology. Biophys J 90:4204–4212
Nemeth A, Kamondi S, Szilagyi A, Magyar C, Kovari Z, Zavodszky P (2002) Increasing the thermal stability of cellulase C using rules learned from thermophilic proteins: a pilot study. Biophys Chem 96:229–241
Petre J, Longin R, Millet J (1981) Purification and properties of an endo-beta-1,4-glucanase from Clostridium thermocellum. Biochimie 63:629–639
Petukhov M, Kil Y, Kuramitsu S, Lanzov V (1997) Insights into thermal resistance of proteins from the intrinsic stability of their alpha-helices. Proteins 29:309–320
Ragone R (2001) Hydrogen-bonding classes in proteins and their contribution to the unfolding reaction. Protein Sci 10:2075–2082
Schwarz WH, Grabnitz F, Staudenbauer WL (1986) Properties of a Clostridium thermocellum endoglucanase produced in Escherichia coli. Appl Environ Microbiol 51:1293–1299
Stemmer WPC (1994) Rapid evolution of a protein in-vitro by DNA shuffling. Nature 370:389–391
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Trevino SR, Schaefer S, Scholtz JM, Pace CN (2007) Increasing protein conformational stability by optimizing β-turn sequence. J Mol Biol 373:211–218
Yao Q, Sun T, Chen G, Liu W (2007) Heterologous expression and site-directed mutagenesis of endoglucanase CelA from Clostridium thermocellum. Biotechnol Lett 29:1243–1247
Zhao HM, Arnold FH (1997) Optimization of DNA shuffling for high fidelity recombination. Nucleic Acids Res 25:1307–1308
Zverlov VV, Kellermann J, Schwarz WH (2005) Functional subgenomics of Clostridium thermocellum cellulosomal genes: Identification of the major catalytic components in the extracellular complex and detection of three new enzymes. Proteomics 5:3646–3653
Acknowledgments
This work was supported by the Program of 100 Distinguished Young Scientists of the Chinese Academy of Sciences, the Province Science Foundation of Sichuan, China (No. 08ZQ026-023 & 2010SZ0128) and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX1-YW-11B2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Purpose of work CelA is one of the major endoglucanases in the cellulosome of thermophilic bacteria Clostridium thermocellum for cellulosic biomass degradation. The stabilizing effect of critical residues of CelA remain have now been studied.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, ZL., Wu, ZL. Mutations from a family-shuffling-library reveal amino acid residues responsible for the thermostability of endoglucanase CelA from Clostridium thermocellum . Biotechnol Lett 32, 1869–1875 (2010). https://doi.org/10.1007/s10529-010-0363-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0363-0