Abstract
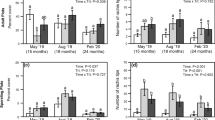
The Australian tree Melaleuca quinquenervia is an invasive weed in wetland systems of Florida, USA. A biological control program targeting M. quinquenervia resulted in the simultaneous release of the gall-fly Fergusonina turneri and the nematode Fergusobia quinquenerviae. Fergusonina (Diptera: Fergusoninidae) flies are gall formers that exploit plants in the Myrtaceae through a mutualistic association with nematodes in the genus Fergusobia (Tylenchida: Neotylenchidae). With a limited number of founding individuals, a risk-spreading release strategy was employed in 2005 by liberating a total of 1996 adult flies across seven locations in southern Florida. However, all release efforts failed to establish a viable population at any of the sites despite variation in location. In an effort to increase founding population size and improve phenological synchrony, 1,432 individual flies and associated nematodes were released within a single M. quinquenervia stand during the early winter months of 2006–2007. The population of F. turneri and F. quinquenerviae persisted at the field site for between two and three generations and, in accordance with the ca. 2-month generation time, emergence of F generation flies peaked in March, May and July 2007. Population growth rate increased with each succeeding generation up to the F3, after which the population went extinct. Both the F1 and F2 generations expanded spatially when compared to the distribution of their respective parental generations. The field population failed to spread after the F2 generation, with F3 generation galls found entirely within the spatial distribution of F2 galls. The release of F. turneri and F. quinquenerviae represent the first obligate mutualism used in weed biological control. Factors contributing to the failure of these species to establish are discussed.

Similar content being viewed by others
References
Andow DA, Kareiva PM, Levin SA, Okubo A (1993) Spread of invading organisms: patterns of spread. In: Kim KC, McPheron BA (eds) Evolution of insect pests: patterns of variation. John Wiley, New York, USA, pp 219–242
Balciunas JK, Burrows DW, Purcell MF (1994) Field and laboratory host ranges of the Australian weevil, Oxyops vitiosa, a potential biological control agent of the paperbark tree, Melaleuca quinquenervia. Biol Control 4:351–360
Barton J, Fowler SV, Gianotti AF, Winks CJ, de Beurs M, Arnold GC, Forrester G (2007) Successful biological control of mist flower (Ageratina riparia) in New Zealand: agent establishment, impact and benefits to the native flora. Biol Control 40:370–385
Beirne BP (1975) Biological control attempts by introductions against pest insects in the field in Canada. Can Entomol 107:225–236
Boland DJ, Brooker MIH, Chippendale GM, Hall N, Hyland BPM, Johnston RD, Kleinig DA, Turner JD (1987) Forest trees of Australia. Nelson Wadsworth, Melbourne, Australia
Butcher PA, Skinner AK, Gardiner CA (2005) Increased inbreeding and inter-species gene flow in remnant populations of the rare Eucalyptus benthamii. Conserv Genet 6:213–226
Center TD, Van TK, Rayachhetry MB, Buckingham GR, Dray FA, Wineriter SA, Purcell MF, Pratt PD (2000) Field colonization of the melaleuca snout beetle (Oxyops vitiosa) in south Florida. Biol Control 19:112–123
Center TD, Purcell MF, Pratt PD, Rayachhetry MB, Tipping PT, Wineriter S, Dray FA Jr (2012) Biological control of Melaleuca quinquenervia—an Everglades invader. BioControl 57:151–165
Cook LG, Morris DC, Edwards RD, Crisp MD (2008) Reticulate evolution in the natural range of the invasive wetland tree species Melaleuca quinquenervia. Mol Phylogenet Evol 47:506–522
Currie GA (1937) Galls on Eucalyptus trees. A new type of association between flies and nematodes. Proc Linn Soc NSW 62:147–176
Davies KA, Giblin-Davis RM (2004) The biology and associations of Fergusobia (Nematoda) from the Melaleuca leucadendra-complex in eastern Australia. Invertebr Syst 18:291–319
Davies KA, Ye W, Giblin-Davis RM, Taylor GS, Scheffer SJ, Thomas WK (2010) The nematode genus Fergusobia (Nematoda: Neotylenchidae): molecular phylogeny, descriptions of clades and associated galls, host plants and Fergusonina fly larvae. Zootaxa 2633:1–66
Dray FA Jr, Center TD, Wheeler GS (2001) Lessons from unsuccessful attempts to establish Spodoptera pectinicornis (Lepidoptera: Noctuidae), a biological control agent of waterlettuce. Biocontrol Sci Tech 11:301–316
Dray FA, Bennett BC, Center TD (2006) Invasion history of Melaleuca quinquenervia (Cav.) S.T. Blake in Florida. Castanea 71:210–225
Frankel OH, Soule ME (1981) Conservation and evolution. Cambridge University Press, Cambridge, UK
Giblin-Davis RM, Davies KA, Williams DS, Center TD (2001a) Cuticular changes in fergusobiid nematodes associated with parasitism of fergusoninid flies. Comp Parasitol 68:242–248
Giblin-Davis RM, Makinson J, Center BJ, Davies KA, Purcell M, Taylor GS, Scheffer SJ, Goolsby J, Center TD (2001b) Fergusobia/Fergusonina-induced shoot bud gall development on Melaleuca quinquenervia. J Nematol 33:239–247
Giblin-Davis RM, Center BJ, Davies KA, Purcell MF, Scheffer SJ, Taylor GS, Goolsby J, Center TD (2004) Histological comparisons of Fergusobia/Fergusonina-induced galls on different Myrtaceous hosts. J Nematol 36:249–262
Goeden RD, Louda SM (1976) Biotic interference with insects imported for weed control. Ann Rev Entomol 21:325–342
Goolsby JA, Makinson J, Purcell M (2000) Seasonal phenology of the gall-making fly Fergusonina sp. (Diptera: Fergusoninidae) and its implications for biological control of Melaleuca quinquenervia. Aust J Entomol 39:336–343
Grevstad FS (1999) Experimental invasions using biological control introductions: the influence of release size on the chance of population establishment. Biol Invasions 1:313–323
Jackson HD, Steane DA, Potts BM, Vaillancourt RE (1999) Chloroplast DNA evidence for reticulate evolution in Eucalyptus (Myrtaceae). Mol Ecol 8:739–751
McKinnon GE, Vaillancourt RE, Steane DA, Potts BM (2004) The rare silver gum, Eucalyptus cordata, is leaving its trace in the organellar gene pool of Eucalyptus globulus. Mol Ecol 13:3751–3762
McKinnon GE, Smith JJ, Potts BM (2010) Recurrent nuclear DNA introgression accompanies chloroplast DNA exchange between two eucalypt species. Mol Ecol 19:1367–1380
Pratt PD, Rayamajhi MB, Van TK, Center TD (2004) Modeling the influence of resource availability on population densities of Oxyops vitiosa, a biological control agent of the invasive tree Melaleuca quinquenervia. Biocontrol Sci Tech 14:51–61
Rayamajhi MB, Van TK, Pratt PD, Center TD (2006) Temporal and structural effects of stands on litter production in Melaleuca quinquenervia dominated wetlands of south Florida. Wetl Ecol Manag 14:303–316
Scheffer SJ, Giblin-Davis RM, Taylor GS, Davies KA, Purcell M, Lewis ML, Goolsby J, Center TD (2004) Phylogenetic relationships, species limits and host specificity of gall-forming Fergusonina flies (Diptera: Fergusoninidae) feeding on Melaleuca (Myrtaceae). Ann Entomol Soc Am 97:1216–1221
Silvers CS, Pratt PD, Ferriter AP, Center TD (2007) T.A.M.E. Melaleuca: a regional approach for suppressing one of Florida’s worst weeds. J Aquat Plant Manag 45:1–8
Silvers CS, Pratt PD, Rayamajhi MB, Center TD (2008) Shoot demographics for melaleuca and impacts of simulated herbivory on vegetative development. J Aquat Plant Manag 46:121–125
Story JM, Anderson NL (1978) Release and establishment of Urophora affinis (Diptera: Tephritidae) on spotted knapweed in western Montana. Environ Entomol 7:445–448
Taylor GS (2004) Revision of Fergusonina Malloch gall flies (Diptera: Fergusoninidae) from Melaleuca (Myrtaceae). Invertebr Syst 18:251–290
Taylor GS, Head ER, Davies KA (2004) Gall flies (Diptera: Fergusoninidae) on Myrtaceae: a mutualistic association between flies and nematodes. In: Raman A, Schaefer CW, Withers TM (eds) Biology, ecology and evolution of Gall-inducing arthropods, vol 2. Sci. Publ. Inc., Enfield, USA, pp 641–669
Ye W, Giblin-Davis RM, Davies KA, Purcell M, Scheffer SJ, Taylor GS, Center TD, Morris K, Thomas WK (2007) Molecular phylogenetics and the evolution of host plant associations in the nematode genus Fergusobia (Tylenchida: Fergusobiinae). Mol Phylogenet Evol 45:123–141
Acknowledgments
We thank two anonymous reviewers for comments on earlier versions of the manuscript. Bradley Brown and Jeff Makinson (CSIRO) were instrumental in collecting the biological control agents in Australia and shipping them to quarantine facilities. Gary Taylor (CSIRO) provided taxonomic confirmation of adult F. turneri. We also thank James Lollis and Deah Lieurance for assistance with colony rearing and data collection. We are indebted to Deah Lieurance, Willey Durden, Eileen Pokorny, Jorge Leidi, Kirsten Dyer, Elizabeth Mattison, and Karen Balentine for assistance with surveys at release locations. This research was supported, in part, by grants from the South Florida Water Management District, the Florida Department of Environmental Protection Bureau of Invasive Plant Management, and the USDA Areawide Melaleuca Demonstration Program. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity employer and provider.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: John Scott.
Rights and permissions
About this article
Cite this article
Pratt, P.D., Blackwood, S., Wright, S.A. et al. The release and unsuccessful establishment of the Melaleuca biological control agent Fergusonina turneri and its mutualistic nematode Fergusobia quinquenerviae . BioControl 58, 553–561 (2013). https://doi.org/10.1007/s10526-013-9505-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-013-9505-3