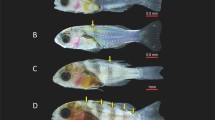
Abstract
Juvenile fish intensively reared under controlled conditions with formulated dry diets often differ in body shape from those fed natural food. These differences, initially invisible by eye and harmless to the fish, over the course of rearing may develop into severe, irreversible body deformities, which can adversely influence fish performance, welfare and production effectiveness in aquaculture. Early detection of the development of deformities makes it possible to implement a procedure to stop or mitigate them. The aim of this study was to create a simple, quick, reliable and safe morphometric method for the early detection of subtle abnormalities in juvenile fish, based solely on basic analysis of digital photographs of individual fish. Three of the six analysed shape variables detected small, yet highly significant (P < 10−6), differences in body shape between ide juveniles fed a formulated dry diet and those fed natural food. The proportion of the caudal length to the fork length (CLFL) was lower, and the first spiny ray of the caudal fin (angle of the first spiny ray of the upper part of the caudal fin (CRA)) was more bent in fish fed the former diet than those fed the latter. These shape variables (CLFL and the angle CRA) were independent of fish size in both groups. The method described in this study proved to be sufficiently sensitive to detect subtle differences in the body shape of juvenile fish intensively fed different diets. The method is simple and inexpensive and can be easily applied in aquaculture practice.




Similar content being viewed by others
References
Adams DC, Rohlf FJ, Slice DE (2004) Geometric morphometrics: ten years of progress following the “revolution”. Ital J Zool 71:5–16
AL-Harbi AH (2001) Skeletal deformities in cultured common carp Cyprinus carpio L. Asian Fish Sci 14:247–254
Baerwald MR, Petersen JL, Hedrick RP, Schisler GJ, May B (2011) A major effect quantitative trait locus for whirling disease resistance identified in rainbow trout (Oncorhynchus mykiss). Heredity 106:920–926
Beamish RJ, Sweeting RM, Neville CM, Lange KL, Beacham TD, Preikshot D (2012) Wild chinook salmon survive better than hatchery salmon in a period of poor production. Environ Biol Fish 94:135–148
Belk MC, Benson LJ, Rasmussen J, Peck SL (2008) Hatchery-induced morphological variation in an endangered fish: a challenge for hatchery-based recovery efforts. Can J Fish Aquat Sci 65:401–408
Blake RW (2004) Fish functional design and swimming performance. J Fish Biol 65:1193–1222
Boglione C, Costa C (2011) Skeletal deformities and juvenile quality. In: Pavlidis MA, Mylonas CC (eds) Sparidae: biology and aquaculture of Gilthead Sea bream and other species. Wiley-Blackwell, Oxford, pp. 233–294
Boglione C, Gavaia P, Koumoundouros G, Gisbert E, Moren M, Fontagne S, Witten PE (2013a) Skeletal anomalies in reared European fish larvae and juveniles. Part 1: normal and anomalous skeletogenic processes. Rev Aquacult 5(Suppl. 1):99–120
Boglione C, Gisbert E, Gavaia P, Witten PE, Moren M, Fontagne S, Koumoundouros G (2013b) Skeletal anomalies in reared European fish larvae and juveniles. Part 2: main typologies, occurrences and causative factors. Rev Aquacult 5(Suppl. 1):121–167
Boglione C, Gagliardi F, Scardi M, Cataudella S (2001) Skeletal descriptors and quality assessment in larvae and post-larvae of wild-caught and hatchery-reared gilthead sea bream (Sparus aurata L. 1758). Aquaculture 192:1–22
Cahu C, Zambonino Infante J, Takeuchi T (2003) Nutritional components affecting skeletal development in fish larvae. Aquaculture 227:245–258
Carral JM, Garcia V, Celada JD, González R, Sáez-Royuela M, González Á (2014) Effects of different photoperiod conditions on juvenile tench (Tinca tinca L.) under intensive rearing. J. Appl. Ichthyol 30(Suppl. 1):44–49
Dabrowski K (1984) The feeding of fish larvae: present “state of art” and perspectives. Reprod Nutr Dev 24:807–833
Dabrowski K, Hinterleitner S, Sturmbauer C, El-Fiky N, Wieser W (1988) Do carp larvae require vitamin C? Aquaculture 72:295–306
Deschamps M-H, Kacem A, Ventura R, Courty G, Haffray P, Meunier FJ, Sire J-Y (2008) Assessment of “discreet” vertebral abnormalities, bone mineralization and bone compactness in farmed rainbow trout. Aquaculture 279:11–17
Divanach P, Boglione C, Menu B, Koumoundouros G, Kentouri M, Cataudella S (1996) Abnormalities in finfish mariculture: an overview of the problem, causes and solutions. In: Chatain B, Saroglia M, Sweetman J, Lavens P (eds) Seabass and seabream culture: problems and prospects. European Aquaculture Society, Oostende, pp. 45–66
Divanach P, Papandroulakis N, Anastasiadis P, Koumoundouros G, Kentouri M (1997) Effect of water currents on the development of skeletal deformities in sea bass (Dicentrarchus labrax L.) with functional swimbladder during postlarval and nursery phase. Aquaculture 156:145–155
Fernández I, Gisbert E (2010) Senegalese sole bone tissue originated from chondral ossification is more sensitive than dermal bone to high vitamin A content in enriched Artemia. J Appl Ichthyol 26:344–349
Fernández I, Gisbert E (2011) The effect of vitamin A on flatfish development and skeletogenesis: a review. Aquaculture 315:34–48
Foster K, Bower L, Piller K (2015) Getting in shape: habitat-based morphological divergence for two sympatric fishes. Biol J Linn Soc 114:152–162
Fulton CJ (2007) Swimming speed performance in coral reef fishes: field validations reveal distinct functional groups. Coral Reefs 26:217–228
Haas TC, Blum MJ, Heins DC (2010) Morphological responses of a stream fish to water impoundment. Biol Lett 6:803–806
Hanson KC, Hasler CT, Suski CD, Cooke SJ (2007) Morphological correlates of swimming activity in wild largemouth bass (Micropterus salmoides) in their natural environment. Comp Biochem Physiol A Mol Integr Physiol 148:913–920
Hard JJ, Berejikian BA, Tezak EP, Schroder SL, Knudsen CM, Parker LT (2000) Evidence for morphometric differentiation of wild and captively reared adult coho salmon: a geometric analysis. Environ Biol Fish 58:61–73
Harris MP, Henke K, Hawkins MB, Witten PE (2014) Fish is fish: the use of experimental model species to reveal causes of skeletal diversity in evolution and disease. J Appl Ichthyol 30:616–629
Huntingford FA, Adams C, Braithwaite VA, Kadri S, Pottinger TG, Sandøe P, Turnbull JF (2006) Current issues in fish welfare. J Fish Biol 68:332–372
Kamiński R, Kamler E, Wolnicki J, Sikorska J, Wałowski J (2010) Condition, growth and food conversion in barbel, Barbus barbus (L.) juveniles under different temperature/diet combinations. J Therm Biol 35:422–427
Kamiński R, Korwin-Kossakowski M, Kusznierz J, Myszkowski L, Stanny LA, Wolnicki J (2005) Response of a juvenile cyprinid, lake minnow Eupallasella perenurus (Pallas), to different diets. Aquacult Int 13:479–486
Kamiński R, Sikorska J, Wolnicki J (2016) Diet and water temperature affect growth and body deformities in juvenile tench Tinca tinca (L.) reared under controlled conditions. Aquacult. Res. doi:10.1111/are.12974.
Kamler E, Wolnicki J (2006) The biological background for the production of stocking material of 11 European rheophilic cyprinids. A review. Arch Hydrobiol Suppl Large Rivers 16:667–687
Kamler E, Myszkowski L, Kamiński R, Korwin-Kossakowski M, Wolnicki J (2006) Does overfeeding affect tench Tinca tinca juveniles? Aquacult Int 14:99–111
Kamler E, Wolnicki J, Kamiński R, Sikorska J (2008) Fatty acid composition, growth and morphological deformities in juvenile cyprinid, Scardinius erythrophthalmus fed formulated diet supplemented with natural food. Aquaculture 278:69–76
Karahan B, Chatain B, Chavanne H, Vergnet A, Bardon A, Haffray P, Dupont-Nivet M, Vandeputte M (2013) Heritabilities and correlations of deformities and growth-related traits in the European sea bass (Dicentrarchus labrax L) in four different sites. Aquac Res 44:289–299
Koumoundouros G, Divanach P, Kentouri M (2001) The effect of rearing conditions on development of saddleback syndrome and caudal fin deformities in Dentex dentex (L.). Aquaculture 200:285–304
Lall SP (2002) The minerals. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Elsevier Science, San Diego, CA, pp. 259–308
Lall SP, Lewis-McCrea LM (2007) Role of nutrients in skeletal metabolism and pathology in fish—an overview. Aquaculture 267:3–19
Latremouille DN (2003) Fin erosion in aquaculture and natural environments. Rev Fish Sci 11:315–335
Lauder GV (2000) Function of the caudal fin during locomotion in fishes: kinematics, flow visualization, and evolutionary patterns. Am Zool 40:101–122
Lauder GV (2006) Locomotion. In: Evans DH, Claiborne JB (eds) The physiology of fishes. CRC Press, Taylor and Francis Group, Boca Raton, FL, pp. 33487–32742
Li D, Hu W, Wang Y, Zhu Z, Fu C (2009) Reduced swimming abilities in fast-growing transgenic common carp Cyprinus carpio associated with their morphological variations. J Fish Biol 74:186–197
Matsuoka M (2003) Comparison of meristic variations and bone abnormalities between wild and laboratory-reared red sea bream. Jpn Agric Res Q 37:21–30
Moore ABM (2015) Morphological abnormalities in elasmobranchs. J Fish Biol 87:465–471
Myszkowski L (2013) Compensatory growth, condition and food utilization in barbel Barbus barbus juveniles reared at different feeding periodicities with a dry diet. J Fish Biol 82:347–353
Myszkowski L, Kamiński R, Quiros M, Stanny LA, Wolnicki J (2002) Dry diet-influenced growth, size variability, condition and body deformities in juvenile crucian carp Carassius carassius L. reared under controlled conditions. Arch Pol Fish 10:51–61
Myszkowski L, Kamler E, Kwiatkowski S (2010) Weak compensatory growth makes short-term starvation an unsuitable technique to mitigate body deformities of Tinca tinca juveniles in intensive culture. Rev Fish Biol Fish 20:381–388
Noble C, Cañon Jones HA, Damsgård B, Flood MJ, Midling KØ, Roque A, Sæther B-S, Cottee SY (2012) Injuries and deformities in fish: their potential impacts upon aquacultural production and welfare. Fish Physiol Biochem 38:61–83
Puvanendran V, Calder-Crewe C, Brown JA (2009) Vertebral deformity in cultured Atlantic cod larvae: ontogeny and effects on mortality. Aquac Res 40:1653–1660
Rennert B, Kohlmann K, Hack H (2003) A performance test with five different strains of tench (Tinca tinca L.) under controlled warm water conditions. J Appl Ichthyol 19:161–164
Sikorska J (2009) Metody przeciwdziałania negatywnym skutkom żywienia starterami młodocianych ryb karpiowatych w warunkach kontrolowanych. [Methods for counteracting negative effects of intensive feeding of juvenile cyprinid fish with dry starter feeds under controlled conditions.]. Ph.D. Thesis, Inland Fisheries Institute in Olsztyn, p.121 [In Polish].
Sikorska J (2012) Zróżnicowana reakcja młodocianych ryb karpiowatych na intensywne żywienie paszą w warunkach kontrolowanych. [Different reaction of juvenile cyprinid fish on intensive feeding with dry diet under controlled conditions.]. In: Zakęś Z, Demska-Zakęś K, Kowalska A (eds) Wylęgarnictwo organizmów wodnych—osiągnięcia, wyzwania i perspektywy [Hatchery procedures of water organisms—achievements, challenges and prospects]. Wydawnictwo IRS, Olsztyn, pp. 179–185 [In Polish]
Webb PW (1984) Body form, locomotion and foraging in aquatic vertebrates. Am Zool 24:107–120
Wimberger PH (1992) Plasticity of fish body shape. The effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biol J Linn Soc 45:197–218
Wolnicki J, Myszkowski L, Korwin-Kossakowski M, Kamiński R, Stanny LA (2006) Effects of different diets on juvenile tench, Tinca tinca (L.) reared under controlled conditions. Aquacult Int 14:89–98
Acknowledgments
This work was conducted in the Inland Fisheries Institute (IFI) in Olsztyn, Poland, Statute Project S-001 with IFI approval to perform experiments on animals, ID Number 0056, decision of the Ministry of Education and Science No. 24/2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korwin-Kossakowski, M., Myszkowski, L. & Kamiński, R. A simple method to detect body morphological abnormalities in juvenile cyprinid fish—a case study on ide Leuciscus idus . Aquacult Int 25, 915–925 (2017). https://doi.org/10.1007/s10499-016-0084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0084-z