Abstract
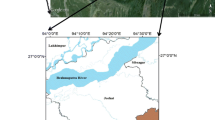
The arsenic (As) concentration in porewaters of the unsaturated (vadose) zone of a watershed located in the Chaco-Pampean Plain of Argentina was investigated. A water displacement method using carbon tetrachloride was applied to the sediments in order to obtain the water samples, which could not be obtained by a simple high-speed centrifugation method. The CD-MUSIC surface complexation model was applied to calculate arsenate adsorption on sediments, arsenate concentration in porewaters in contact with the sediments and effects of carbonate. Ferrihydrite was considered to represent the active adsorbing material in the sediments. Therefore, proton adsorption (surface charge) data and arsenate adsorption isotherms obtained with a synthetic ferrihydrite were used to calibrate the CD-MUSIC model. Arsenate and carbonate concentrations in the studied porewaters were positively correlated. The model was able to predict As concentration within a factor of two in most samples. Carbonate affects As concentration by competing with arsenate species for adsorption sites on the mineral surface. As it occurs with groundwater samples of the saturated zone in many aquifers, this article shows for the first time that adsorption–desorption processes also seem to control As concentration in oxic porewaters of the unsaturated zone.







Similar content being viewed by others
References
Anawar HM, Akai J, Komaki K, Terao H, Yoshioka T, Ishizuka T, Safiullah S, Kato K (2003) Geochemical occurrence of arsenic in groundwater of Bangladesh: sources and mobilization processes. J Geochem Explor 77:109–131
Anawar HM, Akai J, Sakugawa H (2004) Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere 54:753–762
Antelo J, Avena M, Fiol S, López R, Arce F (2005) Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite–water interface. J Colloid Interface Sci 285:476–486
Antelo J, Fiol S, Pérez C, Mariño S, Arce F, Gondar D, López R (2010) Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model. J Colloid Interface Sci 347:112–119
Appelo CAJ, Van der Weiden MJJ, Tournassat C, Charlet L (2002) Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ Sci Technol 36:3096–3103
Arai Y, Sparks DL (2001) ATR–FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite–water interface. J Colloid Interface Sci 241:317–326
Bargar JR, Kubicki JD, Reitmeyer R, Davis JA (2005) ATR–FTIR spectroscopic characterization of coexisting carbonate surface complexes on hematite. Geochim Cosmochim Acta 69:1527–1542
Biswas A, Gustafsson JP, Neidhardt H, Halder D, Kundu AK, Chatterjee D, Berner Z, Bhattacharya P (2014) Role of competing ions in the mobilization of arsenic in groundwater of Bengal Basin: insight from surface complexation modeling. Water Res 55:30–39
Bravo O, Blanco M, Amiotti N (2007) Control factors in the segregation of Mollisols and Aridisols of the semiarid–arid transition of Argentina. Catena 70:220–228
Charlet L, Chakraborty S, Appelo CAJ, Roman-Ross G, Nath B, Ansari AA, Lanson M, Chatterjee D, Basu Mallik S (2007) Chemodynamics of an arsenic “hotspot” in a West Bengal aquifer: a field and reactive transport modeling study. Appl Geochem 22:1273–1292
Cornell RM, Schwertmann U (1996) The iron oxides: structure, properties, reactions, occurrence and uses. Wiley, Weinheim
Dzombak DA, Morel FMM (1990) Surface complexation modeling. hydrous ferric oxide. Wiley, New York
Frau F, Biddau R, Fanfani L (2008) Effect of major anions on arsenate desorption from ferrihydrite-bearing natural samples. Appl Geochem 23:1451–1466
Gustafsson JP (2001) Modelling competitive anion adsorption on oxide minerals and an allophane-containing soil. Eur J Soil Sci 52:639–653
Gustafsson JP (2006) Arsenate adsorption to soils: modelling the competition from humic substances. Geoderma 136:320–330
Hammarlund L, Piñones J (2009) Arsenic in geothermal waters of Costa Rica. Master Thesis. Royal Institute of Technology (KTH), Sweden. http://www2.lwr.kth.se/Publikationer/PDF_Files/LWR_EX_09_02.pdf
Harrington R, Hausner DB, Bhandari N, Strongin DR, Chapman KW, Chupas PJ, Middlemiss DS, Grey CP, Parise JB (2010) Investigation of surface structures by powder diffraction: a differential pair distribution function study on arsenate sorption on ferrihydrite. Inorg Chem 49:325–330
Hausner DB, Bhandari N, Pierre-Louis AM, Kubicki JD, Strongin DR (2009) Ferrihydrite reactivity toward carbon dioxide. J Colloid Interface Sci 337:492–500
Hiemstra T, Van Riemsdijk WH (1996) A surface structural approach to ion adsorption: the charge distribution (CD) model. J Colloid Interface Sci 179:488–508
Hiemstra T, Van Riemsdijk WH (1999) Surface structural ion adsorption modeling of competitive binding of oxyanions by metal (hydr)oxides. J Colloid Interface Sci 210:182–193
Hiemstra T, Van Riemsdijk WH (2006) On the relationship between charge distribution, surface hydration and the structure of the interface of metal hydroxides. J Colloid Interface Sci 301:1–18
Hiemstra T, Van Riemsdijk WH (2009) A surface structural model for ferrihydrite: I. Sites related to primary charge, molar mass and mass density. Geochim Cosmochim Acta 73:4423–4436
Hiemstra T, Rahnemaie R, Van Riemsdijk WH (2004) Surface complexation of carbonate on goethite: IR spectroscopy, structure and charge distribution. J Colloid Interface Sci 278:282–290
Hiemstra T, Antelo J, Rahnemaie R, Van Riemsdijk WH (2010) Nanoparticles in natural systems I: the effective reactive surface area of the natural oxide fraction in field samples. Geochim Cosmochim Acta 74:41–58
Hofmann A, Van Beinum W, Meeussen JCL, Kretzschmar R (2005) Sorption kinetics of strontium in porous hydrous ferric oxide aggregates II. Comparison of experimental results and model predictions. J Colloid Interface Sci 283:29–40
Jessen S, Postma D, Larsen F, Nhan PQ, Hoa LQ, Trang PTK, Long TV, Viet PH, Jakobsen R (2012) Surface complexation modeling of groundwater arsenic mobility: results of a forced gradient experiment in a Red River flood plain aquifer, Vietnam. Geochim Cosmochim Acta 98:186–201
Keizer MG, Van Riemsdijk WH (1998) ECOSAT: Equilibrium calculation of speciation and transport, technical report. Department Soil Science and Plant Nutrition. Wageningen Agricultural University, Wageningen
Kosmulski M (2011a) Compilation of PZC and IEP of sparingly soluble metal oxides and hydroxides from literature. Adv Colloid Interface Sci 152:14–25
Kosmulski M (2011b) The pH-dependent surface charging and points of zero charge V. Update. J Colloid Interface Sci 353:1–15
Kubicki J (2005) Comparison of As(III) and As(V) complexation onto Al and Fe-hydroxides. In: O’Day P, Vlassopoulos D, Benning L (eds) Advances in arsenic research: Integration of experimental and observational studies and implications for mitigation, ACS Symposium Series 915. American Chemical Society, Washington
Lenoble V, Deluchat V, Serpaud B, Bollinger J-C (2003) Arsenite oxidation and arsenate determination by the molybdene blue method. Talanta 61:267–276
Loring JS, Sandström MH, Norén K, Persson P (2009) Rethinking arsenate coordination at the surface of goethite. Chem Eur J 15:5063–5072
Luengo C, Puccia V, Avena M (2011) Arsenate adsorption and desorption kinetics on a Fe(III)-modified montmorillonite. J Hazard Mater 186:1713–1719
Martínez-Villegas N, Briones-Gallardo R, Ramos-Leal JA, Avalos-Borja M, Castañón-Sandoval AD, Razo-Flores E, Villalobos M (2013) Arsenic mobility controlled by solid calcium arsenates: a case study in Mexico showcasing a potentially widespread environmental problem. Environ Pollut 176:114–122
McLaren SJ, Kim ND (1995) Evidence for a seasonal fluctuation of arsenic in New Zealand’s longest river and the effect of treatment on concentrations in drinking water. Environ Pollut 90:67–73
Mubarak A, Olsen RA (1976) Immiscible displacement of the soil solution by centrifugation. Soil Sci Soc Am J 40:329–331
Muller K, Ciminelli VST, Dantas MSS, Willscher S (2010) A comparative study of As(III) and As(V) in aqueous solutions and adsorbed on iron oxy-hydroxides by Raman spectroscopy. Water Res 44:5660–5672
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nickson RT, McArthur JM, Ravenscroft P, Burgess WG, Ahmed KM (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Nimick DA, Moore JN, Dalby CE, Savka MW (1998) The fate of geothermal arsenic in the Madison and Missouri Rivers, Montana and Wyoming. Water Resour Res 34:3051–3067
Postma D, Larsen F, Hue NTM, Duc MT, Viet PH, Nhan PQ, Jessen S (2007) Arsenic in groundwater of the Red River floodplain, Vietnam: controlling geochemical processes and reactive transport modelling. Geochim Cosmochim Acta 71:5054–5071
Robinson B, Outred H, Brooks R, Kirkman J (1995) The distribution and fate of arsenic in the Waikato River System, North Island, New Zealand. Chem Speciat Bioavail 7:89–96
Schwertmann U, Cornell RM (2000) Iron oxides in the laboratory: preparation and characterization. Wiley, Weinheim
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour, and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smedley PL, Nicolli HB, Macdonald DMJ, Barros AJ, Tullio JO (2002) Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl Geochem 17:259–284
Smedley PL, Kinniburgh DG, Macdonald DMJ, Nicolli HB, Barros AJ, Tullio JO, Pearce JM, Alonso MS (2005) Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Appl Geochem 20:989–1016
Stachowicz M (2007) Solubility of arsenic in multi-component systems. From the microscopic to the macroscopic scale. Ph.D. Thesis. Wageningen University, Wageningen
Stachowicz M, Hiemstra T, Van Riemsdijk WH (2006) Surface speciation of As(III) and As(V) in relation to charge distribution. J Colloid Interface Sci 302:62–75
Stachowicz M, Hiemstra T, Van Riemsdijk WH (2007) Arsenic-bicarbonate interaction on goethite particles. Environ Sci Technol 41:5620–5625
Stachowicz M, Hiemstra T, Van Riemsdijk WH (2008) Multi-competitive interaction of As(III) and As(V) oxyanions with Ca2+, Mg2+, PO4 3−, and CO3 2− ions on goethite. J Colloid Interface Sci 320:400–414
Stollenwerk KG (2002) Geochemical processes controlling transport of arsenic in groundwater: a review of adsorption. In: Welch AH, Stollenwerk AG (eds) Arsenic in ground water. Geochemistry and occurrence. Springer, New York, pp 67–100
Stollenwerk KG, Breit GN, Welch AH, Yount JC, Whitney JW, Foster AL, Uddin MN, Majumder RK, Ahmed N (2007) Arsenic attenuation by oxidized aquifer sediments in Bangladesh. Sci Total Environ 379:133–150
Swartz CH, Blute NK, Badruzzman B, Ali A, Brabander D, Jay J, Besancon J, Islam S, Hemond HF, Harvey CF (2004) Mobility of arsenic in a Bangladesh aquifer: inferences from geochemical profiles, leaching data, and mineralogical characterization. Geochim Cosmochim Acta 68:4539–4557
Villalobos M, Leckie JO (2000) Carbonate adsorption onto goethite under closed and open CO2 conditions. Geochim Cosmochim Acta 64:3787–3802
Waychunas GA, Davis JA, Fuller CC (2005) Geometry of sorbed arsenate on ferrihydrite and crystalline FeOOH: re-evaluation of EXAFS results and topological factors in predicting sorbate geometry and evidence for monodentate complexes. Geochim Cosmochim Acta 59:3655–3661
Zeng H, Fisher B, Giammar D (2008) Individual and competitive adsorption of arsenate and phosphate to a high surface-area iron oxide-based sorbent. Environ Sci Technol 42:147–152
Acknowledgments
This work was financed by CONICET, SECyT-Argentina and SECyT-UNS. Olga Pieroni is thanked for her help with infrared measurement. MA and FL are members of CONICET.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
[Hexahidrita170910.MDI] |
SCAN: 20.0/79.976/0.024/1(sec), Cu, I(max) = 41.0, 09/17/10 12:18p |
PEAK: 27-pts/Parabolic filter, Threshold = 3.0, Cutoff = 0.1 %, BG = 3/1.0, Peak-top = Summit |
Intensity = Counts, 2T(0) = 0.0(deg), Wavelength to computed-spacing = 1.54059A (Cu/K-alpha1) |
# | 2-Theta | d(Å) | Height | Height % | Phase ID | d(Å) | I % | (hkl) | 2-Theta | Delta |
|---|---|---|---|---|---|---|---|---|---|---|
Peak ID report | ||||||||||
1 | 21.104 | 4.2063 | 34.5 | 85.9 | ||||||
2 | 25.737 | 3.4587 | 31.3 | 77.8 | ||||||
3 | 33.032 | 2.7096 | 39.3 | 97.8 | ||||||
4 | 33.776 | 2.6516 | 39.0 | 96.9 | ||||||
5 | 34.856 | 2.5719 | 40.2 | 100.0 | ||||||
6 | 35.912 | 2.4986 | 38.1 | 94.7 | Fe5O7(OH)4H2… | 2.5000 | 100.0 | (110) | 35.892 | −0.020 |
7 | 36.752 | 2.4434 | 34.9 | 86.9 | ||||||
8 | 40.136 | 2.2449 | 21.8 | 54.2 | ||||||
9 | 40.474 | 2.2269 | 22.1 | 55.0 | ||||||
10 | 41.072 | 2.1958 | 22.3 | 55.5 | Fe5O7(OH)4H2… | 2.2100 | 80.0 | (200) | 40.797 | −0.275 |
11 | 46.399 | 1.9554 | 19.6 | 48.7 | Fe5O7(OH)4H2… | 1.9600 | 80.0 | (113) | 46.284 | −0.115 |
12 | 50.600 | 1.8025 | 17.0 | 42.3 | ||||||
13 | 51.776 | 1.7642 | 15.6 | 38.8 | ||||||
14 | 54.128 | 1.6930 | 18.2 | 45.2 | ||||||
15 | 55.353 | 1.6584 | 19.7 | 49.0 | ||||||
16 | 57.268 | 1.6074 | 20.0 | 49.8 | ||||||
17 | 59.048 | 1.5631 | 20.9 | 52.0 | ||||||
18 | 61.160 | 1.5141 | 22.8 | 56.6 | Fe5O7(OH)4H2… | 1.5100 | 70.0 | (115) | 61.345 | 0.185 |
19 | 61.832 | 1.4993 | 24.1 | 60.0 | ||||||
20 | 62.959 | 1.4751 | 25.9 | 64.5 | Fe5O7(OH)4H2… | 1.4800 | 80.0 | (106) | 62.728 | −0.231 |
21 | 63.945 | 1.4547 | 23.7 | 58.9 | ||||||
22 | 65.168 | 1.4304 | 18.9 | 47.1 | ||||||
23 | 65.504 | 1.4238 | 16.3 | 40.6 | ||||||
24 | 67.088 | 1.3940 | 14.3 | 35.5 | ||||||
25 | 68.312 | 1.3720 | 12.0 | 29.9 | ||||||
26 | 69.200 | 1.3565 | 14.5 | 36.1 | ||||||
27 | 69.992 | 1.3431 | 11.5 | 28.5 | ||||||
28 | 71.576 | 1.3172 | 12.0 | 29.9 | ||||||
29 | 73.208 | 1.2918 | 11.0 | 27.4 | ||||||
30 | 73.734 | 1.2839 | 14.4 | 35.8 | ||||||
31 | 75.152 | 1.2632 | 10.6 | 26.3 | ||||||
32 | 76.544 | 1.2436 | 10.7 | 26.6 | ||||||
Line shifts of individual phases: 00-029-0712 > Ferrihydrite–Fe5O7(OH)4H2O < 2T(0) = 0.0, d/d(0) = 1.0> | ||||||||||
Rights and permissions
About this article
Cite this article
Puccia, V., Limbozzi, F. & Avena, M. Arsenic in Porewaters of the Unsaturated Zone of an Argentinean Watershed: Adsorption and Competition with Carbonate as Important Processes that Regulate its Concentration. Aquat Geochem 21, 513–534 (2015). https://doi.org/10.1007/s10498-015-9271-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-015-9271-1