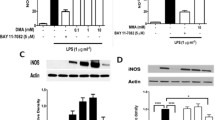
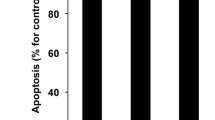
Abstract
Miscarriage caused by Gram-negative bacteria infecting the female genital tract is one of the most common complications of human pregnancy. Intraperitoneal administration of LPS to 7-days pregnant mice induces embryo resorption after 24 h. Here, we show that LPS induced apoptosis on uterine explants from 7-days pregnant mice and that CB1 receptor was involved in this effect. On the other hand, heparin has been widely used for the prevention of pregnancy loss in women with frequent miscarriage with or without thrombophilia. Besides its anticoagulant properties, heparin exerts anti-inflammatory, immunomodulatory and anti-apoptotic effects. Here, we sought to investigate whether the administration of heparin prevented LPS-induced apoptosis in uterine explants from 7-days pregnant mice. We found that heparin enhanced cell survival in LPS-treated uterine explants and that this effect was mediated by increasing uterine FAAH activity. Taken together, our results point towards a novel mechanism involved in the protective effects of heparin.







Similar content being viewed by others
Abbreviations
- AA:
-
Arachidonic acid
- AEA:
-
Anandamide
- CB1:
-
Cannabinoid receptor type 1
- CB2:
-
Cannabinoid receptor type 2
- eCS:
-
Endocannabinoid system
- FAAH:
-
Fatty acid amide hydrolase
- IL:
-
Interleukin
- LMWH:
-
Low molecular weight heparins
- LPS:
-
Lipopolysaccharide
- m-AEA:
-
R(+)-methanandamide
- PBMC:
-
Peripheral blood mononuclear cells
- TUNEL assay:
-
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin DNA-nick end labeling assay
References
Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW (2016) The role of infection in miscarriage. Hum Reprod Update 22(1):116–133. doi:10.1093/humupd/dmv041
Hay P (2004) Bacterial vaginosis and miscarriage. Curr Opin Infect Dis 17:41–44
Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371(9606):75–84. doi:10.1016/S0140-6736(08)60074-4
Gonçalves LF, Chaiworapongsa T, Romero R (2002) Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 8(1):3–13
Aisemberg J, Vercelli C, Wolfson M, Salazar AI, Osycka-Salut C, Billi S, Ribeiro ML, Farina M, Franchi AM (2010) Inflammatory agents involved in septic miscarriage. Neuroimmunomodulation 17:150–152
Ogando DG, Paz D, Cella M, Franchi AM (2003) The fundamental role of increased production of nitric oxide in lipopolysaccharide-induced embryonic resorption in mice. Reproduction 125(1):95–110
Vercelli CA, Aisemberg J, Billi S, Cervini M, Ribeiro ML, Farina M, Franchi AM (2009) Anandamide regulates lipopolysaccharide-induced nitric oxide synthesis and tissue damage in the murine uterus. Reprod Biomed Online 18(6):824–831
Wolfson ML, Correa F, Leishman E, Vercelli C, Cymeryng C, Blanco J, Bradshaw HB, Franchi AM (2015) Lipopolysaccharide-induced murine embryonic resorption involves changes in endocannabinoid profiling and alters progesterone secretion and inflammatory response by a CB1-mediated fashion. Mol Cell Endocrinol 411:214–222. doi:10.1016/j.mce.2015.04.032
Taylor AH, Ang C, Bell SC, Konje JC (2007) The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum Reprod Update 13(5):501–513
Paria BC, Deutsch DD, Dey SK (1996) The uterus is a potential site for anandamide synthesis and hydrolysis: differential profiles of anandamide synthase and hydrolase activities in the mouse uterus during the periimplantation period. Mol Reprod Dev 45(2):183–192
Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK (1997) Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci USA 94:4188–4192
Paria BC, Dey SK (2000) Ligand-receptor signaling with endocannabinoids in preimplantation embryo development and implantation. Chem Phys Lipids 108(1–2):211–220
Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A (2000) Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet 355(9212):1326–1329
Habayeb OM, Taylor AH, Finney M, Evans MD, Konje JC (2008) Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA 299(10):1135–1136
Fonseca BM, Correia-da-Silva G, Teixeira NA (2009) Anandamide-induced cell death: dual effects in primary rat decidual cell cultures. Placenta 30(8):686–692. doi:10.1016/j.placenta.2009.05.012
Contassot E, Tenan M, Schnüriger V, Pelte MF, Dietrich PY (2004) Arachidonylethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol Oncol 93(1):182–188
Wolfson ML, Aisemberg J, Salazar AI, Rubio APD, Vercelli CA, Franchi AM (2013) Progesterone reverts LPS-reduced FAAH activity in murine peripheral blood mononuclear cells by a receptor-mediated fashion. Mol Cell Endocrinol 381(1–2):97–105. doi:10.1016/j.mce.2013.07.020
Shriver Z, Sundaram M, Venkataraman G, Fareed J, Linhardt R, Biemann K, Sasisekharan R (2000) Cleavage of the antithrombin III binding site in heparin by heparinases and its implication in the generation of low molecular weight heparin. Proc Natl Acad Sci USA 97(19):10365–10370
Monien S, Kadecki O, Baumgarten S, Salama A, Dörner T, Kiesewetter H (2009) Use of heparin in women with early and late miscarriages with and without thrombophilia. Clin Appl Thromb Hemost 15(6):636–644. doi:10.1177/1076029609335501
Mastrolia SA, Mazor M, Holcberg G, Leron E, Beharier O, Loverro G, Erez O (2015) The physiologic anticoagulant and anti-inflammatory role of heparins and their utility in the prevention of pregnancy complications. Thromb Haemost 113(6):1236–1246. doi:10.1160/TH14-10-0848
Mousavi S, Moradi M, Khorshidahmad T, Motamedi M (2015) Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci 2015:507151. doi:10.1155/2015/507151
Hills FA, Abrahams VM, González-Timón B, Francis J, Cloke B, Hinkson L, Rai R, Mor G, Regan L, Sullivan M, Lam EW, Brosens JJ (2006) Heparin prevents programmed cell death in human trophoblast. Mol Hum Reprod 12(4):237–243
Bose P, Black S, Kadyrov M, Weissenborn U, Neulen J, Regan L, Huppertz B (2005) Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol 192(1):23–30
Di Simone N, Di Nicuolo F, Castellani R, Veglia M, Tersigni C, Silano M, Tritarelli A, Scambia G, Marana R (2012) Low-molecular-weight heparins induce decidual heparin-binding epidermal growth factor-like growth factor expression and promote survival of decidual cells undergoing apoptosis. Fertil Steril 97(1):169.e1–177.e1. doi:10.1016/j.fertnstert.2011.10.021
Sarrazin S, Lamanna WC, Esko JD (2011) Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3(7) (pii: a004952). doi:10.1101/cshperspect.a004952
Nelson SM, Greer IA (2008) The potential role of heparin in assisted conception. Hum Reprod Update 14(6):623–645. doi:10.1093/humupd/dmn031
Aisemberg J, Vercelli C, Billi S, Ribeiro ML, Ogando D, Meiss R, McCann SM, Rettori V, Franchi AM (2007) Nitric oxide mediates prostaglandins’ deleterious effect on lipopolysaccharide-triggered murine fetal resorption. Proc Natl Acad Sci USA 104(18):7534–7539
Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM (2013) Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PLoS One 8(2):e56161. doi:10.1371/journal.pone.0056161
Maccarrone M, Finazzi-Agró A (2003) The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ 10:946–955. doi:10.1038/sj.cdd.4401284
De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V (1998) The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci USA 95:8375–8380
Melck D, Rueda D, Galve-Roperh I, De Petrocellis L, Guzman M, Di Marzo V (1999) Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett 463:235–240
Eichele K, Ramer R, Hinz B (2009) R(+)-methanandamide-induced apoptosis of human cervical carcinoma cells involves a cyclooxygenase-2-dependent pathway. Pharm Res 26(2):346–355. doi:10.1007/s11095-008-9748-3
Orellana-Serradell O, Poblete CE, Sanchez C, Castellón EA, Gallegos I, Huidobro C, Llanos MN, Contreras HR (2015) Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol Rep 33(4):1599–1608. doi:10.3892/or.2015.3746
Gustafsson K, Sander B, Bielawski J, Hannun YA, Flygare J (2009) Potentiation of cannabinoid-induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Mol Cancer Res 7(7):1086–1098. doi:10.1158/1541-7786.MCR-08-0361
Maldonado R, de Fonseca FR (2002) Cannabinoid addiction: behavioral models and neural correlates. J Neurosci 22(9):3326–3331
Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR (2005) The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci 25(23):5645–5650
Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, Goldberg SR (2008) Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology 33(12):2870–2877
Costa MA, Fonseca BM, Teixeira NA, Correia-da-Silva G (2015) The endocannabinoid anandamide induces apoptosis in cytotrophoblast cells: involvement of both mitochondrial and death receptor pathways. Placenta 36(1):69–76. doi:10.1016/j.placenta.2014.10.011
Gustafsson K, Wang X, Severa D, Eriksson M, Kimby E, Merup M, Christensson B, Flygare J, Sander B (2008) Expression of cannabinoid receptors type 1 and type 2 in non-Hodgkin lymphoma: growth inhibition by receptor activation. Int J Cancer 123(5):1025–1033. doi:10.1002/ijc.23584
Fonseca BM, Correia-da-Silva G, Teixeira NA (2013) The endocannabinoid anandamide induces apoptosis of rat decidual cells through a mechanism involving ceramide synthesis and p38 MAPK activation. Apoptosis 18(12):1526–1535. doi:10.1007/s10495-013-0892-9
Callén L, Moreno E, Barroso-Chinea P, Moreno-Delgado D, Cortés A, Mallol J, Casadó V, Lanciego JL, Franco R, Lluis C, Canela EI, McCormick PJ (2012) Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem 287(25):20851–20865. doi:10.1074/jbc.M111.335273
Kleyer J, Nicolussi S, Taylor P, Simonelli D, Furger E, Anderle P, Gertsch J (2012) Cannabinoid receptor trafficking in peripheral cells is dynamically regulated by a binary biochemical switch. Biochem Pharmacol 83(10):1393–1412. doi:10.1016/j.bcp.2012.02.014
Trabucco E, Acone G, Marenna A, Pierantoni R, Cacciola G, Chioccarelli T, Mackie K, Fasano S, Colacurci N, Meccariello R, Cobellis G, Cobellis L (2009) Endocannabinoid system in first trimester placenta: low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta 30(6):516–522. doi:10.1016/j.placenta.2009.03.015
Pierangeli SS, Chen PP, Raschi E, Scurati S, Grossi C, Borghi MO, Palomo I, Harris EN, Meroni PL (2008) Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost 34(3):236–250. doi:10.1055/s-0028-1082267
Liu Z, Wang L, Dong Z, Pan J, Zhu H, Zhang Z, Ma X (2015) Heparin inhibits lipopolysaccharide-induced inflammation via inducing caveolin-1 and activating the p38/mitogen-activated protein kinase pathway in murine peritoneal macrophages. Mol Med Rep 12(3):3895–3901. doi:10.3892/mmr.2015.3807
Spratte J, Meyer zu Schwabedissen H, Endlich N, Zygmunt M, Fluhr H (2013) Heparin inhibits TNF-α signaling in human endometrial stromal cells by interaction with NF-κB. Mol Hum Reprod 19(4):227–236. doi:10.1093/molehr/gas060
Girardi G, Redecha P, Salmon JE (2004) Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med 10(11):1222–1226
Ceccarelli M, Bani D, Cinci L, Nistri S, Uliva C, Ragazzo E, Vannacci A, Manoni M, Gori AM, Abbate R, Gensini GF, Masini E (2009) Anti-inflammatory effects of low molecular weight heparin derivative in a rat model of carrageenan-induced pleurisy. J Cell Mol Med 13(8B):2704–2712. doi:10.1111/j.1582-4934.2009.00658.x
Gutiérrez G, Sarto A, Berod L, Canellada A, Gentile T, Pasqualini S, Margni RA (2004) Regulation of interleukin-6 fetoplacental levels could be involved in the protective effect of low-molecular weight heparin treatment on murine spontaneous abortion. Am J Reprod Immunol 51(2):160–165
Maccarrone M, De Petrocellis L, Bari M, Fezza F, Salvati S, Di Marzo V, Finazzi-Agrò A (2001) Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch Biochem Biophys 393(2):321–328
Pasquier E, de Saint Martin L, Bohec C, Chauleur C, Bretelle F, Marhic G, Le Gal G, Debarge V, Lecomte F, Denoual-Ziad C, Lejeune-Saada V, Douvier S, Heisert M, Mottier D (2015) Enoxaparin for prevention of unexplained recurrent miscarriage: a multicenter randomized double-blind placebo-controlled trial. Blood 125(14):2200–2205. doi:10.1182/blood-2014-11-610857
Acknowledgments
We thank Ramona Morales and Maximiliano Cella for their excellent technical support. This work was supported by Agencia Nacional para la Promoción Científica y Tecnológica (PICT 2010/0813 and PICT 2013/0097), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 2012/0061). Finally, we would like to thank the animal care technicians Vet. Marcela Márquez and Daniel González for their excellent care of the animals used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salazar, A.I., Vercelli, C., Schiariti, V. et al. Heparin exerts anti-apoptotic effects on uterine explants by targeting the endocannabinoid system. Apoptosis 21, 965–976 (2016). https://doi.org/10.1007/s10495-016-1269-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1269-7