Abstract
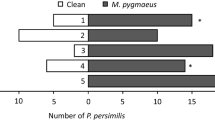
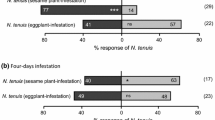
The predatory mite, Phytoseiulus persimilis (Acari: Phytoseiidae), uses plant volatiles (i.e., airborne chemicals) triggered by feeding of their herbivorous prey, Tetranychus urticae (Acari: Tetranychidae), to help locate prey patches. The olfactory response of P. persimilis to prey-infested plants varies in direct relation to the population growth pattern of T. urticae on the plant; P. persimilis responds to plants until the spider mite population feeding on a plant collapses, after which infested plants do not attract predators. It has been suggested that this represents an early enemy-free period for T. urticae before the next generation of females is produced. We hypothesize that the mechanism behind the diminished response of predators is due to extensive leaf damage caused by T. urticae feeding, which reduces the production of volatiles irrespective of the collapse of T. urticae population on the plant. To test this hypothesis we investigated how the response of P. persimilis to prey-infested plants is affected by: 1) initial density of T. urticae, 2) duration of infestation, and 3) corresponding leaf damage due to T. urticae feeding. Specifically, we assessed the response of P. persimilis to plants infested with two T. urticae densities (20 or 40 per plant) after 2, 4, 6, 8, 10, 12 or 14 days. We also measured leaf damage on these plants. We found that predator response to T. urticae-infested plants can be quantified as a function of mite-days, which is a cumulative measure of the standing adult female mite population sampled and summed over time. That is, response to volatiles increased with increasing numbers of T. urticae per plant or with the length of time plant was infested by T. urticae, at least as long at the leaves were green. Predatory mites were significantly attracted to plants that were infested for 2 days with only 20 spider mites. This suggests that the enemy-free period might only provide a limited window of opportunity for T. urticae because relatively low numbers of T. urticae per plant can attract predators. Leaf damage also increased as a function of mite-days until the entire leaf was blanched. T. urticae populations decreased at this time, but predator response to volatiles dropped before the entire leaf was blanched and before the T. urticae population decreased. This result supports our hypothesis that predator response to plant volatiles is linked to and limited by the degree of leaf damage, and that the quantitative response to T. urticae populations occurs only within a range when plant quality has not been severely compromised.



Similar content being viewed by others
References
Bell WJ (1984) Chemo-orientation in walking insects. In: Bell WJ, Cardé RT (eds) Chemical ecology of insects. Chapman and Hall Ltd., London, pp 93–124
Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990a) Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol 16:3091–3118
Dicke M, Van der Maas KJ, Takabayashi J, Posthumus MA (1990b) Learning affects response to volatiles allelochemicals by predatory mites. Proc Exp Appl Entomol NEV (Amsterdam) 1:31–36
Dicke M, van Beek TA, Posthumus MA, Ben Dom N, van Bokhoven H, de Groot AE (1990c) Isolation and identification of volatile kairomone that affects acarine predator-prey interactions: involvement of host plant in its production. J Chem Ecol 16:381–396
Dicke M (1994) Local and systemic production of volatile herbivore-induced terpenoids: their role in plant-carnivore mutualism. J Plant Physiol 143:465–472
Dicke M, Takabayashi J, Posthumus MA, Schutte C, Krips OE (1998) Plant-phytoseiid interactions mediated by herbivore-induced plant volatiles: variation in production of cues and in responses of predatory mites. Exp Appl Acarol 22 1998:311–33
Gols R Roosjen M, Dijkman H, Dicke M (2003) Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and low spider mite infestation. J Chem Ecol 29:2651–2666
Guerin L, Stroup WW (2000) A simulation study to evaluate PROC MIXED analysis of repeated measures data. Proceedings of the 12th Annual Conference on applied statistics in agriculture, Manhattan, KS: Kansas State University, pp 170–203
Hussey NW, Parr WJ (1963) The effect of glasshouse red spider mites (Tetranychus urticae Koch) on the yield of cucumbers. J Hort Sci 38:42–45
Janssen A (1999) Plants with spider-mite prey attract more predatory mites than clean plants under greenhouse conditions. Entomol Exp Appl 90:191–198
Jia F, Margolies DC, Boyer JE, Charlton RE (2002) Genetic variation among foraging traits in inbred lines of a predatory mite. Heredity 88:371–379
Lewis WJ, Martin WR (1990) Semiochemicals for use with parasitoids: status and future. J Chem Ecol 16:3067–3089
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS System for mixed models. SAS Institute Inc, Cary, NC
Maeda T, Takabayashi J (2001) Production of herbivore-induced plant volatiles and their attractiveness to Phytoseiulus persimilis (Acari: Phytoseiidae) with changes of Tetranychus urticae (Acari: Tetranychidae) density on a plant. Appl Entomol Zool 36(1):47–52
Mayland HJ (1998) Effects of prey-induced plant volatiles on search behaviors of the predatory mite, Phytoseiulus persimilis. M.S. thesis, Kansas State University, Manhattan, KS, 63 pp
Nachman G, Zemek R (2002) Interactions in a tritrophic acarine predator-prey metapopulation system III: effects of Tetranychus urticae (Acari: Tetranychidae) on host plant condition. Exp Appl Acarol 25:27–42
Potting RPJ, Vet LEM, Dicke M (1995) Host microhabitat location by stem-borer parasitoid Cotesia flavipes: the role of herbivore volatiles and locally and systemically induced plant volatiles. J Chem Ecol 21:525–539
Roland J (1990) Parasitoid aggregation: chemical ecology and population dynamics. In: Mackauer M, Ehler LE, Roland J (eds) Critcal issues in biological control. Intercept, Andover, Hants, UK, pp 185–211
Röse USR, Manukian A, Heath RR, Tumlinson JH (1996) Volatile semiochemicals released from undamaged cotton leaves – a systemic response of living plants to caterpillar damage. Plant Physiol 111:487–495
Sabelis MW (1981) Biological control of two-spotted spider mite using phytoseiid predators. Part I: Modelling the predator-prey interaction at the individual level. Agricultural research reports 910, Pudoc, Wageningen, Netherlands, 242 pp
Sabelis MW, van de Baan HE (1983) Location of distant spider mite colonies by phytoseiid predators: demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Entomol Exp Appl 33:303–314
Sabelis MW, Vermaat JE, Groeneveld A (1984) Arrestment responses of the predatory mite, Phytoseiulus persimilis, to steep odour gradients of kairomones. Physiol Entomol 9:437–446
Sabelis MW, Dicke M (1985) Long-range dispersal and searching behavior. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control. Elsevier, Ámsterdam, pp 141–159
Sabelis MW, van Baalen M, Bruin J, Egas M, Janssen VAA, Janssen A, Pels B (1999) The evolution of overexpoitation and mutualism in plant-herbivore-predator interactions and its impact on population dynamics. In: Hawkins BA, Cornell HV (eds) Theoretical approaches to biological control. Cambridge University Press, Cambridge, England, pp 259–282
SAS Institute (2001) SAS user’s guide: statistics, version 8.2 SAS Institute, Cary NC
Takabayashi J, Dicke M, Posthumus MA (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2:1–6
Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol 20:1329–1354
Takabayashi J, Dicke M (1996) Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci 1:109–113
Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253
Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92:4169–4174
Turlings TCJ, Tumlinson JH (1992) Systemic release of chemical signals by herbivore-injured corn. Proc Natl Acad Sci USA 89:8399–8402
van Lenteren JC, Woets J (1988) Biological and integrated pest control in greenhouses. Ann Rev Entomol 33:239–269
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Ann Rev Entomol 37:141–172
Walde SJ, Nachman G (1999) Dynamics of spatially structured spider mite populations. In: Hawkins BA, Cornell HV (eds) Theoretical approaches to biological control. Cambridge University Press, Cambridge, UK, pp 163–189
Acknowledgements
We thank Xiaoli Wu, Lessando Gontijo and Nick Timmons for helping with mite inoculations, and James Campbell for review of an earlier draft. Voucher specimens were deposited in the Kansas State University Museum of Entomological and Prairie Arthropod Research as Lot Number 135 (Tetranychus urticae) and Lot Number 154 (Phytoseiulus persimilis). This manuscript is Contribution no. 06–345-J from the Kansas Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nachappa, P., Margolies, D.C., Nechols, J.R. et al. Phytoseiulus persimilis response to herbivore-induced plant volatiles as a function of mite-days. Exp Appl Acarol 40, 231–239 (2006). https://doi.org/10.1007/s10493-006-9043-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-006-9043-0