Abstract
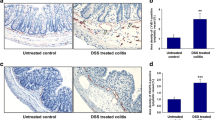
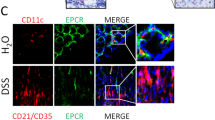
Chronic intestinal inflammation is associated with pathological angiogenesis that further amplifies the inflammatory response. Vascular endothelial growth factor (VEGF), is a major angiogenic cytokine that has been implicated in chronic colitis and inflammatory bowel diseases. Endoglin (CD105), a transforming growth factor-β superfamily co-receptor expressed on endothelial and some myeloid cells, is a modulator of angiogenesis involved in wound healing and potentially in resolution of inflammation. We showed previously that Endoglin heterozygous (Eng +/−) mice subjected to dextran sodium sulfate developed severe colitis, abnormal colonic vessels and high tissue VEGF. We therefore tested in the current study if treatment with a monoclonal antibody to VEGF could ameliorate chronic colitis in Eng +/− mice. Tissue inflammation and microvessel density (MVD) were quantified on histological slides. Colonic wall thickness, microvascular hemodynamics and targeted MAdCAM-1+ inflamed vessels were assessed in vivo by ultrasound. Mediators of angiogenesis and inflammation were measured by Milliplex and ELISA assays. Colitic Eng +/− mice showed an increase in intestinal inflammation, MVD, colonic wall thickness, microvascular hemodynamics and the number of MAdCAM-1+ microvessels relative to colitic Eng +/+ mice; these parameters were all attenuated by anti-VEGF treatment. Of all factors up-regulated in the inflamed gut, granulocyte colony-stimulating factor (G-CSF) and amphiregulin were further increased in colitic Eng +/− versus Eng +/+ mice. Anti-VEGF therapy decreased tissue VEGF and inflammation-induced endoglin, IL-1β and G-CSF in colitic Eng +/− mice. Our results suggest that endoglin modulates intestinal angiogenic and inflammatory responses in colitis. Furthermore, contrast-enhanced ultrasound provides an excellent non-invasive imaging modality to monitor gut angiogenesis, inflammation and responses to anti-angiogenic treatment.







Similar content being viewed by others
References
Abraham C, Cho JH (2009) Inflammatory bowel disease. N Engl J Med 361(21):2066–2078. doi:10.1056/NEJMra0804647
Chidlow JH Jr, Shukla D, Grisham MB, Kevil CG (2007) Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol 293(1):G5–G18. doi:10.1152/ajpgi.00107.2007
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358(19):2039–2049. doi:10.1056/NEJMra0706596
Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D (1990) Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med 172(6):1535–1545
Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D (1996) Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87(8):3336–3343
Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92(11):4150–4166
Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101(12):4878–4886. doi:10.1182/blood-2002-07-1956
Bousvaros A, Leichtner A, Zurakowski D, Kwon J, Law T, Keough K, Fishman S (1999) Elevated serum vascular endothelial growth factor in children and young adults with Crohn’s disease. Dig Dis Sci 44(2):424–430
Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K (2001) VEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol 96(3):822–828. doi:10.1111/j.1572-0241.2001.03527.x
Griga T, Voigt E, Gretzer B, Brasch F, May B (1999) Increased production of vascular endothelial growth factor by intestinal mucosa of patients with inflammatory bowel disease. Hepatogastroenterology 46(26):920–923
Coriat R, Mir O, Leblanc S, Ropert S, Brezault C, Chaussade S, Goldwasser F (2011) Feasibility of anti-VEGF agent bevacizumab in patients with Crohn’s disease. Inflamm Bowel Dis 17(7):1632. doi:10.1002/ibd.21545
Tolstanova G, Khomenko T, Deng X, Chen L, Tarnawski A, Ahluwalia A, Szabo S, Sandor Z (2009) Neutralizing anti-vascular endothelial growth factor (VEGF) antibody reduces severity of experimental ulcerative colitis in rats: direct evidence for the pathogenic role of VEGF. J Pharmacol Exp Ther 328(3):749–757. doi:10.1124/jpet.108.145128
Gougos A, Letarte M (1988) Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol 141(6):1925–1933
Lastres P, Bellon T, Cabanas C, Sanchez-Madrid F, Acevedo A, Gougos A, Letarte M, Bernabeu C (1992) Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol 22(2):393–397. doi:10.1002/eji.1830220216
Rokhlin OW, Cohen MB, Kubagawa H, Letarte M, Cooper MD (1995) Differential expression of endoglin on fetal and adult hematopoietic cells in human bone marrow. J Immunol 154(9):4456–4465
St-Jacques S, Cymerman U, Pece N, Letarte M (1994) Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-beta binding protein of endothelial and stromal cells. Endocrinology 134(6):2645–2657
Adam PJ, Clesham GJ, Weissberg PL (1998) Expression of endoglin mRNA and protein in human vascular smooth muscle cells. Biochem Biophys Res Commun 247(1):33–37. doi:10.1006/bbrc.1998.8734
Bourdeau A, Dumont DJ, Letarte M (1999) A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 104(10):1343–1351. doi:10.1172/JCI8088
Torsney E, Charlton R, Parums D, Collis M, Arthur HM (2002) Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res 51(9):464–470
van de Kerkhof PC, Rulo HF, van Pelt JP, van Vlijmen-Willems IM, De Jong EM (1998) Expression of endoglin in the transition between psoriatic uninvolved and involved skin. Acta Derm Venereol 78(1):19–21
Burke JP, Watson RW, Mulsow JJ, Docherty NG, Coffey JC, O’Connell PR (2010) Endoglin negatively regulates transforming growth factor beta1-induced profibrotic responses in intestinal fibroblasts. Br J Surg 97(6):892–901. doi:10.1002/bjs.6996
Jerkic M, Peter M, Ardelean D, Fine M, Konerding MA, Letarte M (2010) Dextran sulfate sodium leads to chronic colitis and pathological angiogenesis in Endoglin heterozygous mice. Inflamm Bowel Dis 16(11):1859–1870. doi:10.1002/ibd.21288
Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G (2006) Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 281(2):951–961. doi:10.1074/jbc.M508199200
Rychak JJ, Graba J, Cheung AM, Mystry BS, Lindner JR, Kerbel RS, Foster FS (2007) Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Mol Imaging 6(5):289–296
Foster FS, Hossack J, Adamson SL (2011) Micro-ultrasound for preclinical imaging. Interface Focus 1(4):576–601. doi:10.1098/rsfs.2011.0037
Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW (2006) Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131(1):117–129. doi:10.1053/j.gastro.2006.04.020
Loupakis F, Falcone A, Masi G, Fioravanti A, Kerbel RS, Del Tacca M, Bocci G (2007) Vascular endothelial growth factor levels in immunodepleted plasma of cancer patients as a possible pharmacodynamic marker for bevacizumab activity. J Clin Oncol 25(13):1816–1818. doi:10.1200/JCO.2006.10.3051
Hlatky L, Hahnfeldt P, Folkman J (2002) Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst 94(12):883–893
Ardelean DS, Jerkic M, Yin M, Peter M, Ngan B, Kerbel RS, Foster FS, Letarte M (2013) Endoglin and activin receptor-like kinase 1 heterozygous mice have a distinct pulmonary and hepatic angiogenic profile and response to anti-VEGF treatment. Angiogenesis. doi:10.1007/s10456-013-9383-4
Shigematsu T, Specian RD, Wolf RE, Grisham MB, Granger DN (2001) MAdCAM mediates lymphocyte-endothelial cell adhesion in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol 281(5):G1309–G1315
Chidlow JH Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB, Kevil CG (2006) Differential angiogenic regulation of experimental colitis. Am J Pathol 169(6):2014–2030. doi:10.2353/ajpath.2006.051021
Metcalf D (1985) The granulocyte-macrophage colony-stimulating factors. Science 229(4708):16–22
Demetri GD, Griffin JD (1991) Granulocyte colony-stimulating factor and its receptor. Blood 78(11):2791–2808
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29(6):313–326. doi:10.1089/jir2008.0027
Khan WI, Motomura Y, Wang H, El-Sharkawy RT, Verdu EF, Verma-Gandhu M, Rollins BJ, Collins SM (2006) Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol 291(5):G803–G811. doi:10.1152/ajpgi.00069.2006
Shao J, Sheng H (2010) Amphiregulin promotes intestinal epithelial regeneration: roles of intestinal subepithelial myofibroblasts. Endocrinology 151(8):3728–3737. doi:10.1210/en.2010-0319
Zhou Y, Lee JY, Lee CM, Cho WK, Kang MJ, Koff JL, Yoon PO, Chae J, Park HO, Elias JA, Lee CG (2012) Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-beta-induced pulmonary fibrosis. J Biol Chem 287(50):41991–42000. doi:10.1074/jbc.M112.356824
Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ (2013) Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 38(2):275–284. doi:10.1016/j.immuni.2012.09.023
Kanayama M, Takahara T, Yata Y, Xue F, Shinno E, Nonome K, Kudo H, Kawai K, Kudo T, Tabuchi Y, Watanabe A, Sugiyama T (2007) Hepatocyte growth factor promotes colonic epithelial regeneration via Akt signaling. Am J Physiol Gastrointest Liver Physiol 293(1):G230–G239. doi:10.1152/ajpgi.00068.2007
Ido A, Numata M, Kodama M, Tsubouchi H (2005) Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J Gastroenterol 40(10):925–931. doi:10.1007/s00535-005-1705-x
Oh K, Iimuro Y, Takeuchi M, Kaneda Y, Iwasaki T, Terada N, Matsumoto T, Nakanishi K, Fujimoto J (2005) Ameliorating effect of hepatocyte growth factor on inflammatory bowel disease in a murine model. Am J Physiol Gastrointest Liver Physiol 288(4):G729–G735. doi:10.1152/ajpgi.00438.2004
Kallincos NC, Xian CJ, Dunbar AJ, Couper RT, Read LC (2000) Cloning of rat betacellulin and characterization of its expression in the gastrointestinal tract. Growth Factors 18(3):203–213
Feagins LA (2010) Role of transforming growth factor-beta in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis 16(11):1963–1968. doi:10.1002/ibd.21281
Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90(2):770–774
Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT (2001) Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest 108(4):601–609. doi:10.1172/JCI12821
Miyazono K, Kusanagi K, Inoue H (2001) Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 187(3):265–276. doi:10.1002/jcp.1080
Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C (2006) Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 130(7):2060–2073. doi:10.1053/j.gastro.2006.03.054
Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ (1997) Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 151(1):97–110
Farkas S, Hornung M, Sattler C, Edtinger K, Steinbauer M, Anthuber M, Schlitt HJ, Herfarth H, Geissler EK (2006) Blocking MAdCAM-1 in vivo reduces leukocyte extravasation and reverses chronic inflammation in experimental colitis. Int J Colorectal Dis 21(1):71–78. doi:10.1007/s00384-004-0709-y
Bachmann C, Klibanov AL, Olson TS, Sonnenschein JR, Rivera-Nieves J, Cominelli F, Ley KF, Lindner JR, Pizarro TT (2006) Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 130(1):8–16. doi:10.1053/j.gastro.2005.11.009
Vermeire S, Ghosh S, Panes J, Dahlerup JF, Luegering A, Sirotiakova J, Strauch U, Burgess G, Spanton J, Martin SW, Niezychowski W (2011) The mucosal addressin cell adhesion molecule antibody PF-00547, 659 in ulcerative colitis: a randomised study. Gut 60(8):1068–1075. doi:10.1136/gut.2010.226548
Chidlow JH Jr, Glawe JD, Pattillo CB, Pardue S, Zhang S, Kevil CG (2011) VEGF(1)(6)(4) isoform specific regulation of T-cell-dependent experimental colitis in mice. Inflamm Bowel Dis 17(7):1501–1512. doi:10.1002/ibd.21525
Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J, Yla-Herttuala S, Fiocchi C, Danese S (2009) VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 136(2):585–595. doi:10.1053/j.gastro.2008.09.064
Chernoguz A, Crawford K, Vandersall A, Rao M, Willson T, Denson LA, Frischer JS (2012) Pretreatment with anti-VEGF therapy may exacerbate inflammation in experimental acute colitis. J Pediatr Surg 47(2):347–354. doi:10.1016/j.jpedsurg.2011.11.028
Borofsky SE, Levine MS, Rubesin SE, Tanyi JL, Chu CS, Lev-Toaff AS (2013) Bevacizumab-induced perforation of the gastrointestinal tract: clinical and radiographic findings in 11 patients. Abdom Imaging 38(2):265–272. doi:10.1007/s00261-012-9913-3
Goessling W, Mayer RJ (2006) Systemic treatment of patients who have colorectal cancer and inflammatory bowel disease. Gastroenterol Clin North Am 35(3):713–727. doi:10.1016/j.gtc.2006.07.006
Ina K, Kusugami K, Hosokawa T, Imada A, Shimizu T, Yamaguchi T, Ohsuga M, Kyokane K, Sakai T, Nishio Y, Yokoyama Y, Ando T (1999) Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in inflammatory bowel disease. J Gastroenterol Hepatol 14(1):46–53
McAlindon ME, Hawkey CJ, Mahida YR (1998) Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut 42(2):214–219
Ludwiczek O, Vannier E, Borggraefe I, Kaser A, Siegmund B, Dinarello CA, Tilg H (2004) Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin Exp Immunol 138(2):323–329. doi:10.1111/j.1365-2249.2004.02599.x
Nemetz A, Nosti-Escanilla MP, Molnar T, Kope A, Kovacs A, Feher J, Tulassay Z, Nagy F, Garcia-Gonzalez MA, Pena AS (1999) IL1B gene polymorphisms influence the course and severity of inflammatory bowel disease. Immunogenetics 49(6):527–531
Guimbaud R, Bertrand V, Chauvelot-Moachon L, Quartier G, Vidon N, Giroud JP, Couturier D, Chaussade S (1998) Network of inflammatory cytokines and correlation with disease activity in ulcerative colitis. Am J Gastroenterol 93(12):2397–2404. doi:10.1111/j.1572-0241.1998.00694.x
Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C (1994) Macrophages and angiogenesis. J Leukoc Biol 55(3):410–422
Mahida YR (2000) The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis 6(1):21–33
Naito Y, Takagi T, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, Yanagisawa R, Inoue K, Takano H, Satoh M, Yoshida N, Okanoue T, Yoshikawa T (2004) Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin-6-deficient mice. Int J Mol Med 14(2):191–196
Sander LE, Obermeier F, Dierssen U, Kroy DC, Singh AK, Seidler U, Streetz KL, Lutz HH, Muller W, Tacke F, Trautwein C (2008) Gp130 signaling promotes development of acute experimental colitis by facilitating early neutrophil/macrophage recruitment and activation. J Immunol 181(5):3586–3594
Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN (2003) IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA 100(5):2645–2650. doi:10.1073/pnas.0437939100
Holzinger C, Weissinger E, Zuckermann A, Imhof M, Kink F, Schollhammer A, Kopp C, Wolner E (1993) Effects of interleukin-1, -2, -4, -6, interferon-gamma and granulocyte/macrophage colony stimulating factor on human vascular endothelial cells. Immunol Lett 35(2):109–117
Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M (2002) G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun 297(4):1058–1061
Miyake M, Goodison S, Urquidi V, Gomes Giacoia E, Rosser CJ (2013) Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Lab Invest 93(7):768–778. doi:10.1038/labinvest.2013.71
Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE (2008) Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem 283(21):14542–14551. doi:10.1074/jbc.M802139200
Kudo T, Matsumoto T, Nakamichi I, Yada S, Esaki M, Jo Y, Ohji Y, Yao T, Iida M (2008) Recombinant human granulocyte colony-stimulating factor reduces colonic epithelial cell apoptosis and ameliorates murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol 43(6):689–697. doi:10.1080/00365520701864627
Dejaco C, Lichtenberger C, Miehsler W, Oberhuber G, Herbst F, Vogelsang H, Gangl A, Reinisch W (2003) An open-label pilot study of granulocyte colony-stimulating factor for the treatment of severe endoscopic postoperative recurrence in Crohn’s disease. Digestion 68(2–3):63–70. doi:http://www.ncbi.nlm.nih.gov/pubmed/14581762
Korzenik JR, Dieckgraefe BK (2005) An open-labelled study of granulocyte colony-stimulating factor in the treatment of active Crohn’s disease. Aliment Pharmacol Ther 21(4):391–400. doi:10.1111/j.1365-2036.2005.02287.x
Zhong C, Qu X, Tan M, Meng YG, Ferrara N (2009) Characterization and regulation of bv8 in human blood cells. Clin Cancer Res 15(8):2675–2684. doi:10.1158/1078-0432.CCR-08-1954
Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N (2009) G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA 106(16):6742–6747. doi:10.1073/pnas.0902280106
Kapur NK, Wilson S, Yunis AA, Qiao X, Mackey E, Paruchuri V, Baker C, Aronovitz MJ, Karumanchi SA, Letarte M, Kass DA, Mendelsohn ME, Karas RH (2012) Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation 125(22):2728–2738. doi:10.1161/CIRCULATIONAHA.111.080002
Acknowledgments
We thank Genentech for kindly providing the G6-31 anti-VEGF antibody; Dr. Wen-Rong Lie from EMD Millipore for the mouse angiogenesis/growth factor magnetic bead panel kit; Lily Morikawa and Napoleon Law from the Pathology Core Centre of the Modeling Human Disease Toronto Centre for Phenogenomics, Toronto, for help with the CD31 staining; Dr. Herman Yeger, for the use of the Olympus microscope; John Sun (VisualSonics, Toronto) for performing the pilot ultrasound measurements in colitic mice. We also thank the reviewers for their insightful comments. This work was supported by Grants from the Canadian Institute of Health Research (CIHR) (MOP-6247) and the Heart and Stroke Foundation of Canada (T5598) to ML, and from the Terry Fox Foundation, VisualSonics Inc. and CIHR (MOP–12164) to FSF. D. S. Ardelean received the CIHR/Canadian Association of Gastroenterology/Abbott fellowship award.
Conflicts of interest
Dr. Foster discloses that he is a consultant to VisualSonics Inc. The other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ardelean, D.S., Yin, M., Jerkic, M. et al. Anti-VEGF therapy reduces intestinal inflammation in Endoglin heterozygous mice subjected to experimental colitis. Angiogenesis 17, 641–659 (2014). https://doi.org/10.1007/s10456-014-9421-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-014-9421-x