Abstract
Actinobacteria are widely distributed in many environments and represent the most important trigger to the occupant respiratory health. Health complaints, including hypersensitivity pneumonitis of the workers, were recorded in a mushroom compost facility (MCF). The studies on the airborne bacteria were carried out to find a possible microbiological source of these symptoms. Culture analysis of compost bioaerosols collected in different location of the MCF was performed. An assessment of the indoor microbial exposure revealed bacterial flora of bioaerosol in the mushroom compost facility represented by Bacillus, Geobacillus, Micrococcus, Pseudomonas, Staphylococcus spp., and actinobacterial strain with white aerial mycelium. The thermotolerant actinobacterial strain of the same morphology was repeatedly isolated from many locations in MCF: air, compost sample, and solid surface in production hall. On the base of complex morphological, chemotaxonomic, and phylogenetic characteristics, the isolate has been classified as Nocardiopsis alba. Dominant position of N. alba in microbial environment of the mushroom compost facility may represent an indicator microorganism in compost bioaerosol. The bioavailability of N. alba in mushroom compost facility creates potential risk for the health of workers, and the protection of respiratory tract and/or skin is strongly recommended.
Similar content being viewed by others
1 Introduction
A harmful occupational and environmental microbial exposure has been recognized in agriculture for decades. Members of the class Actinobacteria are predominant among airborne environmental microorganisms and represent an important trigger to human respiratory health. Airborne spores of Saccharopolyspora, Streptomyces, and Thermoactinomyces genera (Firmicutes) are responsible for hypersensitivity pneumonitis known as farmer’s or mushroom worker’s lung (Zacharisen and Fink 2011; Xu et al. 2002; Moore et al. 2004) and other severe health effects (Kagen et al. 1981; Lacey and Dutkiewicz 1994).
Spore-forming actinobacteria of the Nocardiopsis genus are opportunistic pathogens rarely encountered in clinical practice. Nevertheless, N. dassonvillei is known as etiologic agent of mycetoma (Sindhuphak et al. 1985), skin lesions, alveolitis (Bernatchez and Lebreux 1991), and pulmonary infections (Mordarska et al. 1998). Diagnostic tests based on biochemical reactions applied in clinical microbiology often do not include actinobacteria; therefore, chemotaxonomic analyses are recommended. Chemotaxonomic markers of the genus Nocardiopsis are comprised of a characteristic fatty acid and polar lipids profile with phosphatidylcholine (Lechevalier et al. 1977) and two major glycolipids (Mordarska et al. 1998). The other chemical markers of Nocardiopsis are peptidoglycan component meso-2,6-diaminopimelic acid (meso-DAP) and the major menachinone (MK-10). There are no characteristic sugars in whole-cell hydrolysates.
Here, we describe an actinobacterial strain isolated from the air of a mushroom compost production facility (MCF) in central Poland. The occupational health service had been informed about a number of respiratory disorders among the workers directly involved in a production, as well as a co-owner of the facility employed in the office. The office worker developed hypersensitivity pneumonitis, a chronic respiratory disorder. To find a possible microbiological cause of these afflictions, the airborne bacteria were isolated and cultivated. Actinobacteria were found in many locations of the mushroom compost facility. The predominate thermotolerant aerial mycelium-producing bacterial strain was selected for identification. The complex morphological, chemotaxonomic, and phylogenetic characteristics were performed for the identification of the airborne actinobacterial strain.
2 Materials and methods
2.1 Description of test locations
The composting plant producing compost for mushroom growing was evaluated for environmental bacteriological contamination. The following locations were selected: production and sale halls (number of samples N = 9); laboratory and offices in factory (N = 7); entrance (N = 2); the factory owners’ house, located in a distance of 100 m from factory (N = 9); house entrance (N = 2); the owners’ cars (N = 2). Outdoor air samples (ca 2 km from the mushroom compost facility) were also collected (N = 5).
2.2 Isolation and cultivation of the bacteria
The samples of air, compost, and from surfaces in the MCF were collected during winter months (November–February 2009). Air samples were collected using a MAS-100 Eco Air Sampler (Merck Millipore). Fifty or hundred liters of air was aspirated and propelled onto a 90-mm Petri dish with either TSA medium (Tryptic Soy Agar, Merck, Germany) or YPG medium (yeast extract–peptone glucose medium; yeast extract 10 g L−1; peptone 20 g L−1, glucose 20 g L−1, pH 7.2). Solid media were prepared with the addition of nystatin (0.2 %). Samples were incubated at 37 ± 2 and 55 ± 2 °C for 48 and 72 h. Bacterial colonies were counted and reported as cfu m−3 (colony-forming units per cubic meter).
In parallel, samples were also taken from surfaces such as walls and equipment of the production hall, to confirm the presence of bacteria from air sampling. The surface samples were collected using Envirocheck® Contact Plates (Merck, Germany) with TSA medium. The air and surface samples were taken in 3–5 repetitions, depending on the premise volume. The presence of bacteria was confirmed in compost samples: 1 g of compost was weighed and mixed with 99 ml of sterile saline, and then a series of dilutions were prepared. The samples of surfaces and compost were incubated on TSA medium at 37 ± 2 and 55 ± 2 °C for 48 and 72 h.
The bacteria were identified by macroscopic and microscopic features observation (color, texture, size, dyes), Gram staining, catalase, oxidase test (Microbiologie Bactident Oxydase, Merck, Germany), and using API® tests (bioMérieux, France): API®50 CH (performance of carbohydrate metabolism tests), API®Staph (identification of staphylococci and micrococci), API®20E (identification of Enterobacteriaceae and other non-fastidious Gram-negative bacteria), API®20NE (identification of Gram-negative non-Enterobacteriaceae).
On the basis of the results, one actinobacterial strain with aerial mycelium, which was dominating in each location (air, surface, and compost samples), was chosen and subjected to further taxonomic identification. An isolated airborne actinobacterial strain was deposited in the Polish Collection of Microorganisms as PCM 2702, preserved in 20 % glycerol solution, and frozen at −70 °C.
2.3 Reference bacterial strains
The reference type strains from microbiological collections were used during study: Nocardiopsis alba PCM 2496 (DSM 43377), N. alborubida PCM 2490 (DSM 40465), N. antarcticus PCM 2489 (JCM 6843), N. prasina PCM 2493 (JCM 3336), and N. dassonvillei PCM 2492 (JCM 7437).
2.4 Morphology and pigmentation analyses
Colony morphology and pigmentation were observed on the following media: nutrient agar (NA), glucose–yeast extract–malt extract (GYM agar), medium 79 (peptone 10 g L−1, yeast extract 2 g L−1, casein hydrolysate 2 g L−1, NaCl 6 g L−1, glucose 20 g L−1, pH 7.5), oatmeal agar (ISP medium 3), inorganic salts-starch agar (ISP medium 4), potato dextrose agar (PDA), tryptone-soya agar (TSA), tryptone yeast extract (TYE), yeast extract–malt extract (MYA), yeast extract–peptone glucose (YPG), and cultivated for 7 days at 37 °C (Shirling and Gottlieb 1966).
2.5 Physiological characteristics and biochemical tests
The optimal growth condition for an actinobacterial isolate was checked at 22, 28, 37, 45, 50, and 55 °C on GYM agar, medium 79, nutrient, and blood agar for 7 days.
The tolerance of different NaCl concentrations (0, 3, 5, 10, 15, 20, 25, 30 % w/v) was determined on GYM agar incubated for 7 days at 37 °C.
A full panel of physiological tests: hydrolysis of starch, gelatin, casein, esculin, adenine, hypoxanthine, xanthine, tyrosine; utylization of d-xylose, d-glucose, l-arabinose, l-rhamnose, d-raffinose, d-mannitol, fructose, cellulose, sucrose, and meso-inositol; decomposition of urea was performed (Yassin et al. 1997).
2.5.1 Antibiotic susceptibility
Minimal inhibitory concentration (MIC) of antibiotics was tested against the airborne actinobacteria strain PCM 2702 and N. alba PCM 2496 and N. prasina PCM 2493 using discs of amoxicillin, erythromycin, gentamicin, tetracycline, and vancomycin, in concentration of 5, 10, and 30 µg on GYM agar incubated for 5 days at 37 °C. The amikacin and oflaxacin were studied in concentration of 30 and 5 µg, respectively.
2.6 Chemotaxonomic studies
An airborne actinobacterial isolate PCM 2702 biomass was obtained by cultivation on medium 79 in the orbitally shaken flasks (for the aeration of bacterial suspension), during 48 h at 37 °C. Bacteria were killed in Koch apparatus (1 h, 100 °C), centrifuged at 11,000g (Sigma), and washed twice by PBS and water. One part of biomass was freeze-dried; the remaining part was stored at −70 °C prior to chemotaxonomic analyses.
Chemotaxonomic identification of the airborne actinobacterial isolate was based on the analyses of the whole-cell sugars, DAP isomer in peptidoglycan, the cellular fatty acids, and the polar lipid profile in the dry and wet biomass (Paściak et al. 2007).
Whole-cell sugars and DAP isomers in whole-cell hydrolysates were determined according to Schaal (1985).
Fatty acid analysis of the whole-cell dry biomass was performed after methanolysis in 2 M methanolic HCl at 80 °C (Paściak et al. 2007). Fatty acid methyl esters (FAMES) were analyzed by gas–liquid chromatography coupled with mass spectrometry (Focus GC with ion trap ITQ 700, Thermo) using helium as the carrier gas and a temperature program on Rxi®-5 ms/Restek column (30 m × 0.25 mm ID) of 150–270 °C, 12 °C/min.
Crude lipid for polar lipid analysis was extracted from dry cell mass by chloroform–methanol (2:1, v/v) at 37 °C according to Bligh–Dyer method (Kates 1986). Polar lipids: phospholipids and glycolipids were analyzed by TLC in chloroform–methanol–water solvent system and visualized by Dittmer and Lester, and orcinol reagent, respectively (Mordarska and Paściak 1994).
Mycolic acids were extracted and analyzed by thin-layer chromatography (TLC) according to Embley and Wait (1994).
2.7 Genotypic identification
Chromosomal DNA was prepared using a DNA extraction kit dedicated to Streptomyces (A&A Biotechnology, Poland). The 16S rRNA gene was enzymatically amplified using the universal oligonucleotide primers 16S start (AGAGTTTGATCMTGGCTCAG) and 16S stop (AAGGAGGTGWTCCARCC) as in Chun and Goodfellow (1995). PCR products were purified and sequenced directly (Genomed Joint-Stock Company, Poland); in parallel, the obtained PCR products were cloned into pGEM®-T Easy vector systems and sequenced.
The identification of phylogenetic neighbors was initially carried out by the BLASTN (Altschul et al. 1997) programs against the database of type strains with validly published prokaryotic names (Kim et al. 2012). The 30 sequences with the highest scores were then selected for the calculation of pairwise sequence similarity using global alignment algorithm (Myers and Miller 1988), which was implemented at the EzTaxon server (http://eztaxon-e.ezbiocloud.net/) (Kim et al. 2012).
Phylogenetic trees were reconstructed using MEGA software version 5.05 (Tamura et al. 2011) for the neighbor-joining method with Kimura two-parameter model and minimum-evolution and maximum-parsimony methods (Takahashi and Nei 2000) and PHYML (Guindon et al. 2005) for the maximum-likelihood method. The topologies of the trees were evaluated by bootstrap analysis (Felsenstein 1985) based on 1,000 resamplings (neighbor-joining, minimum-evolution and maximum-parsimony, and maximum-likelihood). 16S rRNA gene sequence similarity was calculated using the EzTaxon server.
3 Results
3.1 Isolation and cultivation of the airborne actinobacteria
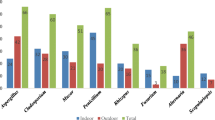
Airborne bacteria were detected in all study locations; actinobacteria were encountered near the compost packing machine and other locations in the mushroom compost facility, as well as in the office/laboratory building and the owner’s house and cars (Table 1). The highest number of actinobacteria was observed in mushroom compost production hall (2.6 × 103 cfu m−3); high values were found also in laboratory and office buildings (Table 1).
The identification results of airborne bacteria in the production hall of the MCF, microorganisms present on solid surfaces, and in compost are shown in Table 2. Only one actinobacterial strain (PCM 2702) was found in bioaerosol, in the compost sample, and on the solid surfaces; four bacterial species (Bacillus sp., Geobacillus thermoglucosidasius, Methylobacterium mesophilicum, and Micrococcus lylae) were present in bioaerosols and in compost. The majority of the strains were isolated from bioaerosol only (Table 2).
Based on these results, the predominant actinobacterial strain in MCF was selected for identification, deposited in Polish Collection of Microorganisms as PCM 2702 and subjected to further cultivation prior to establish its taxonomic position.
3.2 Morphology of the actinobacterial isolate
The airborne actinobacterial strain PCM 2702 was found to be a white aerial mycelium-producing bacterial strain, Gram-positive, aerobic, and non-motile organism with long and branched filaments (Fig. 1). This strain grew well aerobically on nutrient agar, GYM agar, ISP medium 3, TSA, TYE, MYA, and YPG agar. On these media, the substrate mycelium was cream colored and the aerial mycelium was white. The colonies were of irregular edges. No diffusible pigment was produced (Fig. 1).
3.3 Physiological characteristics
The strain PCM 2702 grew up to 45 °C degree with the optimum at 28–37 °C; growth at 22 °C was not observed.
The strain grew in 10 % NaCl (w/v) similarly to the collection strain N. alba PCM 2496. Airborne actinobacterial strain PCM 2702 was catalase-positive, produced nitrate reductase and H2S. Physiological characteristics for type strains of N. alba and N. prasina and airborne strain studied are summarized in Table 3. The airborne isolate PCM 2702 differed from N. alba and N. prasina collection strains in rhamnose utilization as a sole carbon source for growth.
3.4 Antibiotic susceptibility
The MIC was measured for an airborne isolate PCM 2702 and N. alba PCM 2496, N. prasina PCM 2493: amoxicillin ≤10 µg, amikacin ≤30 µg, erythromycin ≤10 µg, gentamicin ≤10 µg, tetracycline ≤10 µg, vancomycin ≤5 µg. Ofloxacin revealed MIC of ≤5 µg for N. alba and airborne isolate PCM 2702 but N. prasina was not susceptible for this antibiotic in concentration studied.
3.5 Chemotaxonomy
Chemotaxonomic studies revealed that strain PCM 2702 had a chemical profile consistent with Nocardiopsis genus. In whole-cell hydrolysates of the strain PCM 2702, meso-DAP was present and no characteristic sugars were detected (III/C cell wall chemotype according to Lechevalier and Lechevalier 1970). The fatty acid profile consisted of branched C14:0, C15:0, C16:0, C17:0, C18:0, and 10-methyl branched C18:0 (TBS) fatty acids (Table 4). Hydroxy fatty acids, including mycolic acids, were not detected. The fatty acid profile of airborne strain PCM 2702 was similar to N. alba PCM 2496 and differed from N. prasina PCM 2493 in terms of TBS content.
The major phospholipids of an airborne isolate PCM 2702 were phosphatidylcholine, phosphatidyl methylethanolamine, diphosphatidylglycerol, phosphatidylglycerol (phospholipid type III, Lechevalier et al. 1977).
Among polar lipids, four major glycolipids were detected; two of them, G1 and G2, characteristic for Nocardiopsis genus (Mordarska et al. 1998), were reported (Fig. 2).
TLC of glycolipids from Nocardiopsis spp.: 1. N. dassonvillei, 2. N. alborubida, 3. Airborne actinobacteria isolate PCM 2702, 4. N. alba, 5. Airborne actinobacteria isolate PCM 2702, 6. N. prasina, and 7. N. antarcticus. Solvent system: chloroform–methanol–water (65:25:4 v/v/v). Detection: orcinol reagent
3.6 16S rRNA sequence analysis
To establish the phylogenetic position of the strain PCM 2702, its 16S rDNA gene sequence was determined in the study (1,525 bp) (GenBank accession no JQ277723). Sequence similarity calculations indicated that the closest relatives of an airborne isolate PCM 2702 were N. alba DSM 43377T (Grund and Kroppenstedt 1990): 99.932 %; N. exhalans ES10.1T (Peltola et al. 2001): 99.105 %; N. prasina DSM 43845T (Yassin et al. 1997): 98.973 %; N. valliformis HBUM 20028T (Yang et al. 2008): 98.953 %; N. lucentensis DSM 44048T (Yassin et al. 1993): 98.904 %. Phylogenetic tree was constructed, showing the nearest phylogenetic relatives of the strain PCM 2702 in Fig. 3.
Strain PCM 2702 revealed 99.93 % 16S rRNA gene sequence similarity to N. alba DSM 43377T. In conclusion, according to genotypic and phenotypic data, an airborne actinobacterial isolate PCM 2702 was proved to represent a new strain of N. alba species.
4 Discussion
Bacterial flora of bioaerosol in the mushroom compost facility studied here is represented by Bacillus, Geobacillus, Micrococcus, Pseudomonas, and Staphylococcus genera. This is rather typical and similar also to other types of compost (e.g., green waste composting plants). In the air samples collected in different compost facilities were identified strains of Bacillus humi, B. niabensis, B. coagulans, Geobacillus thermodenitrificans, Pseudomonas sp. (Karadag et al. 2013), and Leuconostoc pseudomesenteroides (Bru-Adan et al. 2009).
Actinobacteria in bioaerosols of composting plants constitute common flora, especially thermophilic and thermotolerant representatives have been identified: Saccharomonospora, Thermobifida, Saccharopolyspora, Mechercharimyces (Karadag et al. 2013; Le Goff et al. 2011; Schäfer et al. 2013).
Currently, genus Nocardiopsis harbors twelve validly described species and one subspecies (Kroppenstedt and Evtushenko 2006). Of these, the majority were isolated from soils and one (N. compostus) from the bioaerosol in a composting facility (Kämpfer et al. 2002).
The strain PCM 2702 isolated in the mushroom compost facility reported here was a dominant microorganism in bioaerosol. The strain was thermotolerant (as in nearly all Nocardiopsis species) and revealed the optimal growth temperature at 28 °C (Kroppenstedt and Evtushenko 2006).
Physiologic properties and chemotaxonomic studies revealed that the airborne isolate PCM 2702 had a chemical profile consistent with Nocardiopsis genus, i.e., cell wall chemotype III, the fatty acid profile, the phospholipid type, and characteristic glycolipids. Especially, the fatty acid and glycolipid patterns are significant taxonomic markers for Nocardiopsis. 16S rDNA gene sequence analysis revealed that the strain represents N. alba taxon.
Worth to note, N. alba strain was present in the production hall, the office, and the owner’s house, but never detected in outdoor air sampled in a distance of 1 km away from the MCF, suggesting a close relation to microbial environment of the mushroom compost facility.
The availability of large amount of spore-forming actinomycetes in occupational environments can pose potential health problems. The most prevalent form of hypersensitivity pneumonitis is caused mainly by Saccharopolyspora rectivirgula, Thermoactinomyces vulgaris, and T. candidus (McNeil and Brown 1994, Schäfer et al. 2013). Recently, a case of farmer’s lung was described, where positive reaction against Aspergillus fumigatus, Aspergillus terreus, and N. alba was recorded by immunodetection (Imai et al. 2004). So it cannot be ruled out that N. alba caused hypersensitivity pneumonitis in the occupants of the studied buildings.
The role of Nocardiopsis dassonvillei in respiratory tract disorders was reported previously in a study of aerobic actinomycetes involved in human infections in Nigeria, were among 41 patients with bronchopulmonary disorders, N. dassonvillei was detected in two patients (Gugnani et al. 1998), and our group found N. dassonvillei to be the sole etiological agent of severe pulmonary infection (Mordarska et al. 1998). The identification by glycolipid markers was confirmed by DNA/DNA homology studies.
The actinobacterial strain N. alba PCM 2702 isolated in the compost producing facility was strongly dominated in the environment and clearly represented an occupational exposure for the employees. This could be supported by the reporting of one case of hypersensitivity pneumonitis in an office worker and, although not clinically confirmed, chronic headaches and weakness of the others workers and the owner’s family members living in a house in a close distance to the facility.
The bioavailability of N. alba in mushroom compost facility creates potential risk for health for workers, and the protection of respiratory tract and/or skin is strongly recommended. Studies on pathogenicity and allergic properties of N. alba will be continued.
Abbreviations
- PCM:
-
Polish Collection of Microorganisms
- DSM:
-
Deutsche Sammlung von Mikroorganismen
- JCM:
-
Japan Collection of Microorganisms
- MCF:
-
Mushroom compost facility
- YPG:
-
Yeast extract–peptone glucose medium
- cfu:
-
Colony-forming units
- TLC:
-
Thin-layer chromatography
References
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
Bernatchez, H., & Lebreux, E. (1991). Nocardiopsis dassonvillei recovered from a lung biopsy and a possible cause of extrinsic allergic alveolitis. Clinical Microbiology Newsletter, 13, 174–175.
Bru-Adan, V., Wery, N., Moletta-Denat, M., Boiron, P., Delgenes, J. P., & Godon, J. J. (2009). Diversity of Bacteria and fungi in aerosol during screening in green waste composting plant. Current Microbiology, 59, 326–335.
Chun, J., & Goodfellow, M. (1995). A phylogenetic analysis of the genus Nocardia with 16S rRNA gene sequences. International Journal of Systematic Bacteriology, 45, 240–245.
Embley, T. M., & Wait, R. (1994). Structural lipids of eubacteria. In M. Goodfellow & A. G. O’Donnell (Eds.), Chemical methods in prokaryotic systematics (pp. 121–162). Chichester: Wiley.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791.
Grund, E., & Kroppenstedt, R. M. (1990). Chemotaxonomy and numerical taxonomy of the genus Nocardiopsis Meyer 1976. International Journal of Systematic Bacteriology, 40, 5–11.
Gugnani, H. C., Unaogu, I. C., Provost, F., & Boiron, P. (1998). Pulmonary infection due to Nocardiopsis dassonvillei, Gordona sputi, Rhodococcus rhodochrous, and Micromonospora sp. in Nigeria and literature review. Journal de Mycologie Médicale, 8, 21–25.
Guindon, S., Lethiec, F., Duroux, P., & Gascuel, O. (2005). PHYML online—A web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Research, 33, W557–W559.
Imai, K., Ashitani, J., Imazu, Y., Yanagi, S., Sano, A., Tokojima, M., et al. (2004). Farmer’s lung cases of a farmer and his son with high BAL fluid beta-D glucan levels. Nihon Kokyuki Gakkai Zasshi, 42, 1024–1029.
Kagen, S. L., Fink, J. N., Schlueter, D. P., Kurup, V. P., & Fruchtman, R. B. (1981). Streptomyces albus: A new cause of hypersensitivity pneumonitis. Journal of Allergy and Clinical Immunology, 68, 295–299.
Kämpfer, P., Busse, H. J., & Rainey, F. (2002). Nocardiopsis compostus sp. nov., from the atmosphere of a composting facility. International Journal of Systematic and Evolutionary Microbiology, 52, 621–627.
Karadag, D., Özkaya, B., Ölmez, E., Nissilä, M. E., Cakmakci, M., Yildiz, S., et al. (2013). Profiling of bacterial community in a full-scale aerobic composting plant. International Biodeterioration and Biodegradation, 77, 85–90.
Kates, M. (1986). Lipids extraction procedures. In R. H. Burdon & P. H. van Knippenberg (Eds.), Laboratory techniques in biochemistry and molecular biology Vol. 3, part 2, Techniques of lipidology: isolation, analysis and identification of lipids (pp. 100-111). Amsterdam: Elsevier.
Kim, O. S., Cho, Y. J., Lee, K., Yoon, S. H., Kim, M., Na, H., et al. (2012). Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. International Journal of Systematic and Evolutionary Microbiology, 62, 716–721.
Kroppenstedt, R. M., & Evtushenko, L. I. (2006). The family Nocardiopsaceae. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, & E. Stackebrandt (Eds.), The prokaryotes: A handbook on the biology of bacteria (Vol. 3, pp. 754-795). New York: Springer.
Lacey, J., & Dutkiewicz, J. (1994). Bioaerosols and occupational lung disease. Journal of Aerosol Science, 25, 1371–1404.
Le Goff, O., Godon, J. J., Steyer, J. P., & Wery, N. (2011). New specific indicators for qPCR monitoring of airborne microorganisms emitted by composting plants. Atmospheric Environment, 45, 5342–5350.
Lechevalier, M. P., De Bièvre, C., & Lechevalier, H. A. (1977). Chemotaxonomy of aerobic actinomycetes: Phospholipid composition. Biochemical Systematics and Ecology, 5, 249–260.
Lechevalier, M. P., & Lechevalier, H. (1970). Chemical composition as a criterion in the classification of aerobic actinomycetes. International Journal of Systematic Bacteriology, 20, 435–443.
McNeil, M. M., & Brown, J. M. (1994). The medically important aerobic actinomycetes: Epidemiology and microbiology. Clinical Microbiology Reviews, 7, 357–417.
Moore, J. E., Xu, J., Millar, B. C., Elborn, J. S., & Rao, J. R. (2004). Identification of an organism associated with mushroom worker’s lung. Compost Science & Utilization, 12, 192–195.
Mordarska, H., & Paściak, M. (1994). A simple method for differentiation of Propionibacterium acnes and Propionibacterium propionicum. FEMS Microbiology Letters, 123, 325–329.
Mordarska, H., Zakrzewska-Czerwińska, J., Paściak, M., Szponar, B., & Rowiński, S. (1998). Rare, suppurative pulmonary infection caused by Nocardiopsis dassonvillei recognized by glycolipid markers. FEMS Immunology and Medical Microbiology, 21, 47–55.
Myers, E. W., & Miller, W. (1988). Optimal alignments in linear space. Computer Applications in the Biosciences, 4, 11–17.
Paściak, M., Mordarska, H., Szponar, B., & Gamian, A. (2007). Chemotaxonomical methods in the diagnostics of clinical strains causing actinobacterial infections (in Polish). Postepy Higieny i Medycyny Doswiadczalnej, 61, 403–412.
Peltola, J. S., Andersson, M. A., Kämpfer, P., Auling, G., Kroppenstedt, R. M., Busse, H. J., et al. (2001). Isolation of toxigenic Nocardiopsis strains from indoor environments and description of two new Nocardiopsis species, N. exhalans sp. nov. and N. umidischolae sp. nov. Applied and Environmental Microbiology, 67, 4293–4304.
Schaal, K. P. (1985). Identification of clinically significant Actinomycetes and related bacteria using chemical techniques. In M. Goodfellow & D. E. Minnikin (Eds.), Chemical methods in bacterial systematics (pp. 359–381). London: Academic Press.
Schäfer, J., Klug, K., van Kampen, V., & Jäckel, U. (2013). Quantification of Saccharopolyspora rectivirgula in composting plants: Assessment of the relevance of S. rectivirgula. The Annals of Occupational Hygiene, 57, 875–883.
Shirling, E. B., & Gottlieb, D. (1966). Methods for characterization of Streptomyces species. International Journal of Systematic Bacteriology, 16, 313–340.
Sindhuphak, W., Macdonald, E., & Head, E. (1985). Actinomycetoma caused by Nocardiopsis dassonvillei. Archives of Dermatology, 121, 1332–1334.
Takahashi, K., & Nei, M. (2000). Efficiencies of fast algorithms of phylogenetic inference under the criteria of maximum parsimony, minimum evolution, and maximum likelihood when a large number of sequences are used. Molecular Biology and Evolution, 17, 1251–1258.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Xu, J., Rao, J. R., Millar, B. C., Elborn, J. S., Evans, J., Barr, J. G., et al. (2002). Improved molecular identification of Thermoactinomyces spp. associated with mushroom worker’s lung by 16S rDNA sequence typing. Journal of Medical Microbiology, 51, 1117–1127.
Yang, R., Zhang, L. P., Guo, L. G., Shi, N., Lu, Z., & Zhang, X. (2008). Nocardiopsis valliformis sp. nov., an alkaliphilic actinomycete isolated from alkali lake soil in China. International Journal of Systematic and Evolutionary Microbiology, 58, 1542–1546.
Yassin, A. F., Galinski, E. A., Wohlfarth, A., Jahnke, K. D., Schaal, K. P., & Trüper, H. G. (1993). A new actinomycete species, Nocardiopsis lucentensis sp. nov. International Journal of Systematic Bacteriology, 43, 266–271.
Yassin, A. F., Rainey, F. A., Burghardt, J., Gierth, D., Ungerechts, J., Lux, I., et al. (1997). Description of Nocardiopsis synnemataformans sp. nov., elevation of Nocardiopsis alba subsp. prasina to Nocardiopsis prasina comb. nov., and designation of Nocardiopsis antarctica and Nocardiopsis alborubida as later subjective synonyms of Nocardiopsis dassonvillei. International Journal of Systematic Bacteriology, 47, 983–988.
Zacharisen, M. C., & Fink, J. N. (2011). Hypersensitivity pneumonitis and related conditions in the work environment. Immunology and Allergy Clinics of North America, 31, 769–786.
Acknowledgments
The financial support from the Project No. III.B.03 “Improving safety and working conditions,” 2nd stage, Part B, for Central Institute of Labor Protection—National Research Institute, Poland—is gratefully acknowledged.
Conflict of interest
The authors declare they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Paściak, M., Pawlik, K., Gamian, A. et al. An airborne actinobacteria Nocardiopsis alba isolated from bioaerosol of a mushroom compost facility. Aerobiologia 30, 413–422 (2014). https://doi.org/10.1007/s10453-014-9336-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-014-9336-4