Abstract
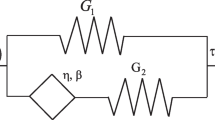
The mechanical loading environment encountered by articular cartilage in situ makes frictional-shear testing an invaluable technique for assessing engineered cartilage. Despite the important information that is gained from this testing, it remains under-utilized, especially for determining damage behavior. Currently, extensive visual inspection is required to assess damage; this is cumbersome and subjective. Tools to simplify, automate, and remove subjectivity from the analysis may increase the accessibility and usefulness of frictional-shear testing as an evaluation method. The objective of this study was to determine if the friction signal could be used to detect damage that occurred during the testing. This study proceeded in two phases: first, a simplified model of biphasic lubrication that does not require knowledge of interstitial fluid pressure was developed. In the second phase, frictional-shear tests were performed on 74 cartilage samples, and the simplified model was used to extract characteristic features from the friction signals. Using support vector machine classifiers, the extracted features were able to detect damage with a median accuracy of approximately 90%. The accuracy remained high even in samples with minimal damage. In conclusion, the friction signal acquired during frictional-shear testing can be used to detect resultant damage to a high level of accuracy.








Similar content being viewed by others
References
Basalo, I. M., N. O. Chahine, M. Kaplun, F. H. Chen, C. T. Hung, and G. A. Ateshian. Chondroitin sulfate reduces the friction coefficient of articular cartilage. J. Biomech. 40:1847–1854, 2007.
Burges, C. J. C. A tutorial on support vector machines for pattern recognition. Data Min. Knowl. Disc. 2:121–167, 1998.
Burnham, K. P., and D. R. Anderson. Multimodel inference understanding AIC and BIC in model selection. Sociol. Methods Res. 33:261–304, 2004.
Caligaris, M., and G. A. Ateshian. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthr. Cartil. 16:1220–1227, 2008.
Caligaris, M., C. E. Canal, C. S. Ahmad, T. R. Gardner, and G. A. Ateshian. Investigation of the frictional response of osteoarthritic human tibiofemoral joints and the potential beneficial tribological effect of healthy synovial fluid. Osteoarthr. Cartil. 17:1327–1332, 2009.
Changzheng, C., S. Changcheng, Z. Yu, and W. Nan. Fault diagnosis for large-scale wind turbine rolling bearing using stress wave and wavelet analysis. ICEMS 3:2239–2244, 2005.
Chung, C., I. E. Erickson, R. L. Mauck, and J. A. Burdick. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng. A 14:1121–1131, 2008.
Forster, H., and J. Fisher. The influence of loading time and lubricant on the friction of articular cartilage. Proc. Inst. Mech. Eng. H 210:109–119, 1996.
Forster, H., and J. Fisher. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proc. Inst. Mech. Eng. H 213:329–345, 1999.
Graindorge, S., and G. Stachowiak. Changes occurring in the surface morphology of articular cartilage during wear. Wear 241:143–150, 2000.
Hanley, J. A., and B. J. McNeil. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36, 1982.
Henderson, J. H., J. F. Welter, J. M. Mansour, C. Niyibizi, A. I. Caplan, and J. E. Dennis. Cartilage tissue engineering for laryngotracheal reconstruction: comparison of chondrocytes from three anatomic locations in the rabbit. Tissue Eng. 13:843–853, 2007.
Jones, A. R., J. P. Gleghorn, C. E. Hughes, L. J. Fitz, R. Zollner, S. D. Wainwright, B. Caterson, E. A. Morris, L. J. Bonassar, and C. R. Flannery. Binding and localization of recombinant lubricin to articular cartilage surfaces. J. Orthop. Res. 25:283–292, 2007.
Krishnan, R., M. Kopacz, and G. A. Ateshian. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J. Orthop. Res. 22:565–570, 2004.
Liu, B. Web Data Mining. Berlin: Springer, 2011.
Lizhang, J., J. Fisher, Z. Jin, A. Burton, and S. Williams. The effect of contact stress on cartilage friction, deformation and wear. Proc. Inst. Mech. Eng. H 225:461–475, 2011.
Martin, I., S. Miot, A. Barbero, M. Jakob, and D. Wendt. Osteochondral tissue engineering. J. Biomech. 40:750–765, 2007.
McGill, R., J. W. Tukey, and W. A. Larsen. Variations of box plots. Am. Stat. 32:12–16, 1978.
Morrell, K. C. Corroboration of in vivo cartilage pressures with implications for synovial joint tribology and osteoarthritis causation. Proc. Natl. Acad. Sci. U.S.A. 102:14819–14824, 2005.
Mu, T., A. K. Nandi, and R. M. Rangayyan. Screening of knee-joint vibroarthrographic signals using the strict 2-surface proximal classifier and genetic algorithm. Comput. Biol. Med. 38:1103–1111, 2008.
Rangayyan, R. M., F. Oloumi, Y. Wu, and S. Cai. Fractal analysis of knee-joint vibroarthrographic signals via power spectral analysis. Biomed. Signal Process. Control 8:23–29, 2013.
Reddy, N. P., B. M. Rothschild, M. Mandal, V. Gupta, and S. Suryanarayanan. Noninvasive acceleration measurements to characterize knee arthritis and chondromalacia. Ann. Biomed. Eng. 23:78–84, 1995.
Robin, X., N. Turck, A. Hainard, N. Tiberti, F. Lisacek, J.-C. Sanchez, and M. Müller. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 12:77, 2011.
Schmidt, T. A., N. S. Gastelum, Q. T. Nguyen, B. L. Schumacher, and R. L. Sah. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 56:882–891, 2007.
Unsworth, A., D. Dowson, and V. Wright. The frictional behavior of human synovial joints—part i: natural joints. J. Lubr. Technol. 97:369–376, 1975.
Wada, Y., M. Enjo, N. Isogai, R. Jacquet, E. Lowder, and W. J. Landis. Development of bone and cartilage in tissue-engineered human middle phalanx models. Tissue Eng. A 15:3765–3778, 2009.
Whitney, G. A., K. Jayaraman, J. E. Dennis, and J. M. Mansour. Scaffold-free cartilage subjected to frictional shear stress demonstrates damage by cracking and surface peeling. J. Tissue Eng. Regen. Med. 2014. doi:10.1002/term.1925.
Whitney, G. A., H. Mera, M. Weidenbecher, A. Awadallah, J. M. Mansour, and J. E. Dennis. Methods for producing scaffold-free engineered cartilage sheets from auricular and articular chondrocyte cell sources and attachment to porous tantalum. Biores. Open Access 1:157–165, 2012.
Acknowledgments
This work was supported by NIH NIDCR grant number R01 DE015322 (J.E.D.), and NIH NIAMS Grant Number P01 AR053622 (J.M.M), and under the Ruth L. Kirschstein National Research Service Award T32 AR007505 from the NIH NIAMS (G.A.W.).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric M. Darling oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Whitney, G.A., Mansour, J.M. & Dennis, J.E. Coefficient of Friction Patterns Can Identify Damage in Native and Engineered Cartilage Subjected to Frictional-Shear Stress. Ann Biomed Eng 43, 2056–2068 (2015). https://doi.org/10.1007/s10439-015-1269-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1269-8