Abstract
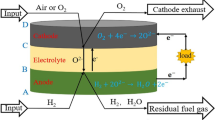
In co-ionic conducting solid oxide fuel cell (SOFC), both oxygen ion (O2-) and proton (H+) can transport through the electrolyte, generating steam in both the anode and cathode. Thus the mass transport phenomenon in the electrodes is quite different from that in conventional SOFC with oxygen ion conducting electrolyte (O-SOFC) or with proton conducting electrolyte (H-SOFC). The generation of steam in both electrodes also affects the concentration overpotential loss and further the SOFC performance. However, no detailed modeling study on SOFCs with co-ionic electrolyte has been reported yet. In this paper, a new mathematical model for SOFC based on co-ionic electrolyte was developed to predict its actual performance considering three major kinds of overpotentials. Ohm’s law and the Butler-Volmer formula were used to model the ion conduction and electrochemical reactions, respectively. The dusty gas model (DGM) was employed to simulate the mass transport processes in the porous electrodes. Parametric simulations were performed to investigate the effects of proton transfer number (t H) and current density (j total) on the cell performance. It is interesting to find that the co-ionic conducting SOFC could perform better than O-SOFC and H-SOFC by choosing an appropriate proton transfer number. In addition, the co-ionic SOFC shows smaller difference between the anode and cathode concentration overpotentials than O-SOFC and H-SOFC at certain t H values. The results could help material selection for enhancing SOFC performance.
Similar content being viewed by others
References
Demin, A., Tsiakaras, P.: Thermodynamic analysis of a hydrogen fed solid oxide fuel cell based on a proton conductor. International Journal of Hydrogen Energy 26, 1103–1108 (2001)
Iwahara, H.: Oxide-ionic and protonic conductors based on perovskite-type oxides and their possible applications. Solid State Ionics 52, 99–104 (1992)
Iwahara, H.: High temperature protonic conductors and their applications. In: Proceedings of International Conference on Advanced Materials, ICA91, Strabourg, France (1992)
Iwahara, H.: High temperature proton conducting oxides and their applications to solid electrolyte fuel cells and steam electrolyzer for hydrogen production. In: Proceedings of the 6th Internal Conference on Solid State Ionics (1998)
Iwahara, H., Yajima, T., Hibino, T., et al.: Performance of solid oxide fuel-cell using proton and oxide-ion mixed conductors based on Bace1-xsmxo3-Alpha. Journal of the Electrochemical Society 140, 1687–1691 (1993)
Huang, J.B., Yuan, J.L., Mao, Z.Q., et al.: Analysis and modeling of novel low temperature SOFC with a co-ionic conducting ceria based composite electrolyte. Journal of Fuel Cell Science and Technology 7, 11–12 (2010)
Bavarian, M., Kevrekidis, I.G., Benziger, J.B., et al.: Modeling and bifurcation analysis of a coionic conducting solid oxide fuel cell. Industrial & Engineering Chemistry Research 52, 3165–3177 (2013)
Demin, A., Tsiakaras, P., Gorbova, E., et al.: A SOFC based on a co-ionic electrolyte. Journal of Power Sources 131, 231–236 (2004)
Demin, A., Gorbova, E., Tsiakaras, P.: High temperature electrolyzer based on solid oxide co-ionic electrolyte: A theoretical model. Journal of Power Sources 171, 205–211 (2007)
Ni, M., Leung, M.K.H., Leung, D.Y.C.: Parametric study of solid oxide fuel cell performance. Energy Conversion and Management 48, 1525–1535 (2007)
Hernández-Pacheco, E., Singh, D., Hutton, P.N., et al.: A macro-level model for determining the performance characteristics of solid oxide fuel cells. Journal of Power Sources 138, 174–186 (2004)
Suwanwarangkul, R., Croiset, E., Fowler, M.W., et al.: Performance comparison of Fick’s, dusty-gas and Stefan-Maxwell models to predict the concentration overpotential of a SOFC anode. Journal of Power Sources 122, 9–18 (2003)
Ni, M., Leung, M.K.H., Leung, D.Y.C.: Theoretical analysis of reversible solid oxide fuel cell based on proton-conducting electrolyte. Journal of Power Sources 177, 369–375 (2008)
Peng, R.R., Wu Y., Yang, L.Z., et al.: Electrochemical properties of intermediate-temperature SOFCs based on proton conducting Sm-doped BaCeO3 electrolyte thin film. Solid State Ionics 177, 389–393 (2006)
Ni, M., Leung, M.K.H., Leung, D.Y.C.: Mathematical modeling of proton-conducting solid oxide fuel cells and comparison with oxygen-ion-conducting counterpart. Fuel Cells 7, 269–278 (2007)
Author information
Authors and Affiliations
Corresponding author
Additional information
The project was supported by Research Grant Council (RGC) of Hong Kong (PolyU 5238/11E).
Rights and permissions
About this article
Cite this article
Zheng, KQ., Ni, M., Sun, Q. et al. Mathematical analysis of SOFC based on co-ionic conducting electrolyte. Acta Mech Sin 29, 388–394 (2013). https://doi.org/10.1007/s10409-013-0040-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-013-0040-3