Abstract
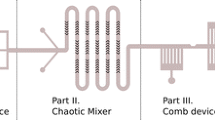
We have designed a microfluidic system that enables both the fabrication of calibrated capsules and the in situ characterization of their mechanical properties. The fabrication setup consists of a double flow-focusing system. A human serum albumin aqueous solution is introduced in the central channel of a first Y-junction. Intercepted by the lateral flows of a hydrophobic phase, it is dispersed into microdroplets. A cross-linking agent is then introduced at a second Y-junction allowing a membrane to form around the droplets. The time of cross-linking is controlled by the length of a wavy channel located downstream of the second junction. A cylindrical microchannel finally enables to deform and characterize the capsules thus formed. The mechanical properties of the capsule membrane are obtained by inverse analysis. The results show that the drop size increases with the flow rate ratio between the central and lateral channels. The mean shear modulus of the capsules fabricated after 23 s of cross-linking is of the order of the surface tension between the two phases indicating that a reaction time of 23 s is too short for an elastic membrane to form around the droplet. When the cross-linking time is increased to 60 s, the microcapsules surface is wrinkled, thus confirming that a solid membrane is formed around the drop. The mean shear modulus of the capsule membrane increases with the cross-linking time, which is in agreement with our previous chemical results and proves that a fine control of the mechanical properties is possible by choosing adequately the control parameters of the system.







Similar content being viewed by others
References
Andry M-C, Edwards-Lévy F, Lévy M-C (1996) Free amino group content of serum albumin microcapsules III: a study at low pH values. Int J Pharm 128:197–202
Anna SL, Bontoux N, Stone HA (2003) Formation of dispersions using ’flow focusing’ in microchannels. Appl Phys Lett 82:364–366
Callewaert M, Millot JM, Lesage J, Laurent-Maquin D, Edwards-Lévy F (2009) Albumin-alginate microspheres: role of structure in binding and release of the krfk peptide. Int J Pharm 366:103–110
Carin M, Barthès-Biesel D, Edwards-Lévy F, Postel C, Andrei DC (2003) Compression of biocompatible liquid-filled HSA-alginate capsules: determination of the membrane mechanical properties. Biotechnol Bioeng 82:207–212
Chu TX, Salsac AV, Leclerc E, Barthès-Biesel D, Wurtz H, Edward-Lévy F (2011) Comparison between measurements of elasticity and free amino group content of ovalbumin microcapsule membranes: discrimination of the cross-linking degree. J Colloid Interf Sci 355:81–88
De Kruif CG, Weinbreck F, De Vries R (2004) Complex coacervation of proteins and anionic polysaccharides. Curr Opin Colloid Interf Sci 9:340–349
Edwards-Lévy F (2011) Microparticulate drug delivery systems based on serum albumin. In: Serum albumin: structure, functions, and health impact. Nova Science
Edwards-Lévy F, Andry M-C, Lévy M-C (1993) Determination of free amino group content of serum albumin microcapsules using trinitrobenzenesulfonic acid: effect of variations in polycondensation pH. Int J Pharm 96:85–90
Edwards-Lévy F, Andry M-C, Lévy M-C (1994) Determination of free amino group content of serum albumin microcapsules: II. Effect of variation time and in terephthaloyl chloride concentration. Int J Pharm 103:253–257
Fery A, Weinkamer R (2007) Mechanical properties of micro and nanocapsules: Single-capsule measurements. Polym Adv Technol 48:7221–7235
Garstecki P, Fuerstman MJ, Stonec HA, Whitesides GM (2006) Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of break-up. Lab Chip 6:437–446
Gautier A, Carpentier B, Dufresne M, Vu Dinh Q, Paulier P, Legallais C (2011) Impact of alginate type and bead diameter on mass transfers and the metabolic activities on encapsulated c3a cells in bioactificial liver applications. Eur Cell Mater 21:94–106
Gibbs BF, Kermasha S, Alli I, Mulligan CN (1999) Encapsulation in the food industry: a review. Int J Food Sc. Nutr 50:213–224
He P, Barthès-Biesel D, Leclerc E (2010) Flow of two immiscible liquids with low viscosity in y shaped microfluidic systems: effect of geometry. Microfluid Nanofluid 9:293–301
Hu X-Q, Salsac A-V, Barthès-Biesel D (2012) Flow of a spherical capsule in a pore with circular or square cross-section. J Fluid Mech 705:176–194
Huang K-S, Liu M-K, Wu C-H, Yen Y-T, Lin Y-C (2007) Calcium alginate microcapsule generation on a microfluidic system fabricated using the optical disk process. J Micromech Microeng 17:1428–1434
Hurteaux R, Edwards-Lévy F, Laurent-Maquin D, Lévy M-C (2005) Coating alginate microspheres with a serum albumin-alginate membrane: application to the encapsulation of a peptide. Eur J Pharm Sci 24:187–197
Kissel T, Maretschek S, Packhaser C, Schnieders J, Seidel N (2006) Microencapsulation techniques for parenteral depot systems and their application in the pharmaceutical industry. In: Benita S (ed) Microencapsulation—methods and industrial applications, 2nd edn. Taylor and Francis
Lefebvre Y, Leclerc E, Barthès-Biesel D, Walter J, Edwards-Lévy F (2008) Flow of artificial microcapsules in microfluidic channels: a method for determining the elastic properties of the membrane. Phys Fluids 20:1–10
Lévy MC, Lefebvre S, Rahmouni M, Andry MC, Manfait M (1991) Fourier transform infrared spectroscopic studies of human serum albumin microcapsules prepared by interfacial cross-linking with terephthaloylchloride: Influence of polycondensation ph on spectra and relation with microcapsule morphology and size. J Pharm Sci 80:578–585
Liu L, Yang J-P, Ju X-J, Xie R, Yang L, Liang B, Chu L-Y (2009) Microfluidic preparation of monodisperse ethyl cellulose hollow microcapsules with non-toxic solvent. J Colloids Interf Sci 336:100–106
Mercadé-Prieto R, Zhang Z (2012) Mechanical characterization of microspheres, capsules, cells and beads: a review. J Microencapsulation 29:277–285
Miyazawa K, Yajima I, Kaneda I, Yanaki T (2000) Preparation of a new soft capsule for cosmetics. J Cosmet Sci 51:239–252
Needham D, Zhelev DV (1996) The mechanochemistry of lipid vesicles examined by micropipet manipulation technique. Surf Sci 62:373–444
Ng Lee J, Park C, Whitesides GM (2003) Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem 75:6544–6554
Nguyen QT, Bendjama Z, Clment R, Ping Z (1999) Poly(dimethylsiloxane) crosslinked in different conditions. Part I: sorption properties in water-ethyl acetate mixtures. Phys Chem 1:2761–2766
Poux M, Canselier J.P (2004) Techniques et appareillage, procédés d’émulsification. Technique de l’ingénieur, Chapitre 3. J2153. Techniques de l’ingénieur
Sawalha H, Schron K, Boom R (2011) Biodegradable polymeric microcapsules: preparation and properties. Chem Eng J 169:1–10
Thorsen T, Roberts RW, Arnold FH, Quake SR (2001) Dynamic pattern formation in a vesicle-generating microfluidic device. Phys Rev Lett 86:4163–4166
Yeh C-H, Zhao Q, Lee S-J, Lin Y-C (2009) Using a t-junction microdluidic chip for monodisperse calcium alginate mircoparticles and encapsulation of nanoparticles. Sensor Actuat A Phys 151:231–236
Yobas L, Martens S, Ong W-L, Ranganathan N (2006) High-performance flow-focusing geometry for spontaneous generation of monodispersed droplets. Lab Chip 6:1073–1079
Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RMA, Walker GC, Kumacheva E (2006) Microfluidic production of biopolymer microcapsules with controlled morphology. J Am Chem Soc 128:12205–12210
Acknowledgments
This work was supported by the Conseil Regional de Picardie (projects μFIEC and MODCAP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, T.X., Salsac, AV., Barthès-Biesel, D. et al. Fabrication and in situ characterization of microcapsules in a microfluidic system. Microfluid Nanofluid 14, 309–317 (2013). https://doi.org/10.1007/s10404-012-1049-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1049-9