Abstract
Purpose
The purpose was to evaluate the long-term efficacy of ranibizumab for the treatment of myopic choroidal neovascularization (CNV) in a clinical setting.
Methods
This was a retrospective, monocentric, noncomparative analysis of 51 eyes of 51 patients with naïve juxtafoveal or subfoveal myopic CNV treated with intravitreal ranibizumab (IVR) on a pro re nata basis for at least 24 months. The patients’ demographic data were recorded, including the best-corrected visual acuity (BCVA) measured with an ETDRS chart, location of the CNV, grade of myopic changes, central foveal thickness (CFT), and number of administered IVR. Outcome measures were to determine the changes in BCVA, identify the factors influencing the visual outcome, compare the best visual gain obtained for each treated eye with the final visual gain, and identify the cause of the relative decline in the visual acuity, when present.
Results
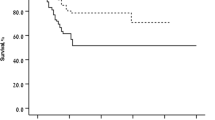
BCVA improved from 58.7 ± 19.0 letters at baseline to 66.3 ± 16.2 letters at the final visit (P = 0.001; mean visual gain: +7.6 ± 15.6 letters). Multivariate analysis did not identify any correlation between the visual gain and age, sex, grade of myopic fundus changes, CNV location, or initial protocol. The mean IVR number was 3.5 ± 2.8 injections (range 1–12; median 3) for a mean follow-up of 39.3 ± 11.3 months (range 24–69). Twenty-one eyes experienced a relative decline in BCVA during the follow-up, which was attributable in 16 cases to myopic atrophic changes.
Conclusions
Intravitreal ranibizumab resulted in long-term efficacy in the treatment of myopic CNV. However, some eyes may present a long-term relative decline in their initial visual gain.


Similar content being viewed by others
References
Xu L, Wang Y, Li Y, Cui T, Li J, Jonas JB. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113:1134.e1-11.
Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006;113:1354–62.
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ. Etiology of choroidal neovascularization in young patients. Ophthalmology. 1996;103:1241–4.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Mimoun G, Tilleul J, Leys A, Coscas G, Soubrane G, Souied EH. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2010;150:692–700.
Heier JS, Brown D, Ciulla T, Abraham P, Bankert JM, Chong S, et al. Ranibizumab for choroidal neovascularization secondary to causes other than age-related macular degeneration: a phase I clinical trial. Ophthalmology. 2011;118:111–8.
Gupta B, Elagouz M, Sivaprasad S. Intravitreal bevacizumab for choroidalneovascularisation secondary to causes other than age-related macular degeneration. Eye (Lond). 2010;24:203–13.
Wang E, Chen Y. Intravitreal anti-vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: systematic review and meta-analysis. Retina. 2013;33:1375–92.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitrealranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83.
Querques G, Azrya S, Martinelli D, Berboucha E, Feldman A, Pece A, et al. Ranibizumab for exudative age-related macular degeneration: 24-month outcomes from a single-centre institutional setting. Br J Ophthalmol. 2010;94:292–6.
Rothenbuehler SP, Waeber D, Brinkmann CK, Wolf S, Wolf-Schnurrbusch U. Effects of ranibizumab in patients with subfoveal choroidal neovascularization attributable to age-related macular degeneration. Am J Ophthalmol. 2009;147:831–7.
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. Ranibizumab and bevacizumab for treatment of neovascular age- related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98.
IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411.
Cohen SY, Dubois L, Tadayoni R, Fajnkuchen F, Nghiem-Buffet S, Delahaye-Mazza C, et al. Results of one-year’s treatment with ranibizumab for exudative age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2009;148:409–13.
Bandukwala T, Muni RH, Schwartz C, Eng KT, Kertes PJ. Effectiveness of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration in a Canadian retina practice: a retrospective review. Can J Ophthalmol. 2010;45:590–5.
Rotsos T, Patel PJ, Chen FK, Tufail A. Initial clinical experience of ranibizumab therapy for neovascular age-related macular degeneration. Clin Ophthalmol. 2010;10:1271–5.
Bloch SB, la Cour M, Sander B, Hansen LK, Fuchs J, Lund-Andersen H, et al. Predictors of 1-year visual outcome in neovascular age-related macular degeneration following intravitreal ranibizumab treatment. Acta Ophthalmol. 2013;91:42–7.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial: VIP report no. 3. Ophthalmology. 2003;110:667–73.
Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial: VIP report no. 1. Ophthalmology. 2001;108:841–52.
Cohen SY. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina. 2009;29:1062–6.
Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens C. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91:1573–81.
Iacono P, Parodi MB, Papayannis A, Kontadakis S, Sheth S, Cascavilla ML, et al. Intravitreal ranibizumab versus bevacizumab for treatment of myopic choroidal neovascularization. Retina. 2012;32:1539–46.
Iacono P, Parodi MB, Papayannis A, Kontadakis S, Sheth S, Bandello F. Intravitreal bevacizumab therapy on an as-per-needed basis in subfoveal choroidal neovascularization secondary to pathological myopia: 2-year outcomes of a prospective case series. Retina. 2011;31:1841–7.
Oishi A, Yamashiro K, Tsujikawa A, Ooto S, Tamura H, Nakata I, et al. Long-term effect of intravitreal injection of anti-VEGF agent for visual acuity and chorioretinal atrophy progression in myopic choroidal neovascularization. Graefes Arch ClinExpOphthalmol. 2013;251:1–7.
Peiretti E, Vinci M, Fossarello M. Intravitreal bevacizumab as a treatment for choroidalneovascularisation secondary to myopia: 4-year study results. Can J Ophthalmol. 2012;47:28–33.
Lai TY, Luk FO, Lee GK, Lam DS. Long-term outcome of intravitreal anti-vascular endothelial growth factor therapy with bevacizumab or ranibizumab as primary treatment for subfoveal myopic choroidal neovascularization. Eye (Lond). 2012;26:1004–11.
Kang HM, Koh HJ. Ocular risk factors for recurrence of myopic choroidal neovascularization: long-term follow-up study. Retina. 2013;33:1613–22.
Franqueira N, Cachulo ML, Pires I, Fonseca P, Marques I, Figueira J, et al. Long-term follow-up of myopic choroidal neovascularization treated with ranibizumab. Ophthalmologica. 2012;227:39–44.
Yang HS, Kim JG, Kim JT, Joe SG. Prognostic factors of eyes with naïve subfoveal myopic choroidal neovascularization after intravitreal bevacizumab. Am J Ophthalmol. 2013;156:1201–21.
Ruiz-Moreno JM, Arias L, Montero JA, Carneiro A, Silva R. Intravitreal anti-VEGF therapy for choroidal neovascularisation secondary to pathological myopia: 4-year outcome. Br J Ophthalmol. 2013;97:1447–50.
Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno-Matsui K, Sharma T, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121:682–92.
Tufail A, Narendran N, Patel PJ, Sivaprasad S, Amoaku W, Browning AC, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology. 2013;120:1944–5.
Acknowledgments
This study was supported by an unrestricted grant from CIL-ASSOC, Association for Research and Education, Paris, France.
Conflicts of interest
S. Y. Cohen, Board membership (Allergan, Bayer, Novartis), Consultant fees (Allergan, Bayer, Bausch and Lomb, Novartis, Thea); S. Nghiem-Buffet, Board membership (Allergan, Bayer, Novartis), Consultant fee (Novartis); T. Grenet, Board membership (Allergan, Novartis); L. Dubois, None; S. Ayrault, None; F. Fajnkuchen, Board membership (Allergan, Novartis), Consultant fee (Bayer); C. Delahaye-Mazza, None; G. Quentel, Board membership (Novartis), Consultant fee (Novartis); and R. Tadayoni, Board membership (Alcon, Novartis, Allergan, Bausch & Lomb, Pfizer, Alimera, Bayer, FCI-Zeiss), Consultant fees (Allergan, DORC, Alcon, Novartis, Takeda, Bausch & Lomb, FCI-Zeiss).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cohen, S.Y., Nghiem-Buffet, S., Grenet, T. et al. Long-term variable outcome of myopic choroidal neovascularization treated with ranibizumab. Jpn J Ophthalmol 59, 36–42 (2015). https://doi.org/10.1007/s10384-014-0363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-014-0363-z