Abstract
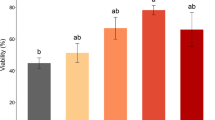
The objective of this study was to determine how the extender and dilution ratio used during centrifugation affect bear spermatozoa quality before and after freezing–thawing. Semen was collected from 15 brown bears by electroejaculation. In experiment 1, semen was divided into five aliquots and diluted using one of the following extenders: Tris-citric-glucose (TCG), Tris-citric-glucose-3% BSA, Tris-citric-glucose-1% egg yolk or CaninePro. In experiment 2, semen was divided into five aliquots and diluted 1:1, 1:4, 1:8 or 1:16 (semen:extender) with Tris-citric-glucose. In both experiments, one aliquot was left undiluted and it was used as a control. All the aliquots were centrifuged at 600×g for 6 min and frozen. Samples were analysed by post-thawing for motility (CASA) and, by flow cytometry, for viability (YO-PRO-1), acrosomal status (PNA-FITC/PI) and mitochondrial status (JC-1). CaninePro rendered the highest motility with respect to the undiluted control (total motility, 53.1% vs. 38.5%, P < 0.001), and CaninePro and TCG significantly increased the percentage of viable and acrosome-intact spermatozoa (43.2 and 43.4, respectively, vs. 39.4, P < 0.05). In experiment 2, dilution 1:4 yielded the highest value of total motility (78.8 vs. 67.2, P < 0.05) and proportion of spermatozoa with intact membrane and acrosome (64.5 vs. 54.4, P < 0.01). In general, diluting 1:4 or 1:8 brown bear semen prior to centrifugation improved the motility and acrosome status of the thawed spermatozoa.


Similar content being viewed by others
References
Aitken RJ, Clarkson JS (1988) Significance of reactive oxygen species and antioxidants in defining of the efficiency of sperm preparation technique. J Androl 9:367–376
Althouse GC, Seager SWJ, Varner DD, Webb GW (1989) Diagnostic aids for the detection of urine in the equine ejaculate. Theriogenology 31:1141–1148
Alvarez JG, Lasso JL, Blasco L, Nuñez RC, Heyner S, Caballero PP, Storey BT (1993) Centrifugation of human spermatozoa induces sublethal damage; separation of human spermatozoa from seminal plasma by a dextran swim-up procedure without centrifugation extends their motile lifetime. Hum Reprod 8(7):1087–1092
Anel L, de Paz P, Alvarez M, Chamorro CA, Boixo JC, Manso A, Gonzalez M, Kaabi M, Anel E (2003) Field and in vitro assay of three methods for freezing ram semen. Theriogenology 60:1293–1308
Anel L, Alvarez M, Martínez-Pastor F, Gomes S, Nicolás M, Mata M, Martínez AF, Borragán S, Anel E, de Paz P (2008) Sperm cryopreservation in brown bear (Ursus arctos): preliminary result. Reprod Domest Anim 43:9–17
Anel L, Gomes-Alves S, Alvarez M, Borragan S, Anel E, Nicolas M, Martinez-Pastor F, de Paz P (2010) Effect of basic factors of extender composition on post-thawing quality of brown bear electroejaculated spermatozoa. Theriogenology. doi:10.1016/j.theriogenology.2010.03.004
Brinsko SP, Crockett EC, Squire EL (2000) Effect of centrifugation and partial removal of seminal plasma on equine spermatozoal motility after cooling and storage. Theriogenology 54:129–136
Cardullo RA, Cone RA (1986) Mechanical immobilization of rat sperm does not exchange their oxygen consumption rate. Biol Reprod 34:820–830
Carvajal G, Cuello C, Ruiz M, Vazquez JM, Martinez EA, Roca F (2004) Effects of centrifugation before freezing on boar sperm cryosurvival. J Androl 25:389–396
Carver DA, Ball BA (2002) Lipase activity in stallion seminal plasma and the effect of lipase on stallion spermatozoa during storage at 5 degrees C. Theriogenology 58(8):1587–1595
Chen LM, Hou R, Zhang ZH, Wang JS, An XR, Chen YF, Zheng HP, Xia GL, Zhang MJ (2007) Electroejaculation and semen characteristics of Asiatic Black bears (Ursus thibetanus). Anim Reprod Sci 101:358–364
Crockett EC, Graham JK, Bruemmer JE, Squires EL (2001) Effect of cooling on equine spermatozoa before freezing on post-thaw motility: preliminary results. Theriogenology 55:793–803
García-Macías V, Martínez-Pastor F, Álvarez M, Paz P, Borragán S, Anel E, Mata M, Anel L (2007) Use of a triple-stain (SYBR-14/PI/MC540) for viability and capacitation assessment in thawed semen from brown bear (Ursus arctos). Reprod Fertil Dev 19(1):239
Ishikawa A, Matsui M, Sakamoto H, Katagiri S, Takahashi Y (2001) Cryopreservation of the semen collected by electroejaculation from the Hokkaido brown bear (Ursus arctos yesoensis). J Vet Sci 64(4):373–376
Katkov II, Mazur P (1998) Influence of centrifugation regimes on motility, yield and cell associations of mouse spermatozoa. J Androl 19:232–241
Katkov II, Ostashko FI (1996) Correlation between electromechanical stability of cytoplasm membrane and cryoresistivity of bovine spermatozoa. Cryobiology 33:680–687
Kim SC, Kim HW (1998) Effects of nitrogenous components of urine on sperm motility: an in vitro study. Int J Androl 21(1):29–33
Kojima E, Tsuruga H, Komatsu T, Murase T, Tsubota TM, Kita I (2001) Characterization of semen collected from beagles and captive Japanese black bears (Ursus thibetanus japonicus). Theriogenology 55:717–731
Makler A, David R, Blumenfeld Z, Better OS (1981) Factors affecting sperm motility. VII. Sperm viability as affected by change of pH and osmolarity of semen and urine specimens. Fertil Steril 36(4):507–511
Martinez-Pastor F, Anel L, Guerra C, Alvarez M, Soler AJ, Garde JJ, Chamorro C, de PP (2006) Seminal plasma improves cryopreservation of Iberian red deer epididymal sperm. Theriogenology 66:1847–1856
Matas C, Decuadro G, Martinez-Miro S, Gadea J (2007) Evaluation of a cushioned method for centrifugation and processing for freezing boar semen. Theriogenology 67:1087–1091
McMahon CG, Abdo C, Incrocci L, Perelman M, Rowland D, Waldinger M, Xin ZC (2004) Disorders of orgasm and ejaculation in men. The Journal of Sexual Medicine 1:58–65
Mortimer D, Goel N, Shu MA (1988) Evaluation of the cell soft automated semen analysis system in a routine laboratory setting. Fertil Steril 50:960–968
Ng SC, Bongso TA, Sathananthan H, Tok VCN, Ratnam SS (1990) Microcentrifugation of human spermatozoa: its effect on fertilization of hamster oocytes after micro-insemination spermatozoal transfer. Hum Reprod 5:209–211
Okano T, Murase T, Tsubota T (2004) Electroejaculation and semen cryopreservation of free-ranging Japanese black bears (Ursus thibetanus japonicus). J Vet Med Sci 66(11):1371–1376
Ollero M, Cebrian-Pérez JA, Muiño-Blando T (1997) Improvement of cryopreserved ram sperm heterogeneity and viability by addition of seminal plasma. J Androl 18(6):732–739
Pellicer-Rubio MT, Magallon T, Combarnous Y (1997) Deterioration of goat sperm viability in milk extenders is due to a bulbourethral 60-kilodalton glycoprotein with triglyceride lipase activity. Biol Reprod 57:1023–1031
Pickett BW, Sullivan JJ, Byers WW, Pace MM, Remmenga EE (1975) Effect of cenrifugation and seminal plasma on motility and fertility of stallion and bull spermatozoa. Fertil Steril 26:167–174
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, ISBN 3-900051-07-0, http://www.R-project.org
Rijsselaere T, Van Soom A, Maes D, de Kruif A (2002) Effect of centrifugation on in vitro survival of fresh diluted canine spermatozoa. Theriogenology 57:1669–1681
Ritar AJ, Salamon S (1982) Effects of seminal plasma and of its removal and of egg yolk in the diluent on the survival of fresh and frozen-thawed spermatozoa of the Angora goat. Aust J Bil Sci 35(3):305–312
Rota A, Milani C, Romagnoli S (2006) Effect of post-thaw dilution whit autologous prostatic fluid on dog semen motility and sperm acrosome status. Theriogenology 67:520–525
Shäfer-Somi S, Kluger S, Knapp E, Klein D, Aurich C (2006) Effects of semen extender and semen processing on motility and viability of frozen-thawed dog spermatozoa. Theriogenology 66(2):173–182
Sieme H, Katila T, Klug E (2004) Effect of semen collection practices on sperm characteristics before and after storage and on fertility of stallions. Theriogenology 61:769–784
Varisli O, Uguz C, Agca C, Agca Y (2009) Effect of various physical stress factors on rat sperm motility, acrosome and plasma membrane integrity. J Androl 30:75–86
Waite JA, Love CC, Brinsko SP, Teague SR, Salazar JL Jr, Mancill SS, Varner DD (2008) Factors impacting equine sperm recovery rate and quality following cushioned centrifugation. Theriogenology 70:704–714
Way AL, Lester CG, Killian GJ (2000) Effects of accessory sex gland fluid on viability, capacitation, and the acrosome reaction of cauda epididymal bull spermatozoa. J Androl 21:213–219
Acknowledgements
The authors thank to M. A. Marañon and other keepers of Cabárceno Park (Cantabria, Spain) for their help in managing bears; and to S. Gomes and E. Anel for their help in the acquisition and analysis of the samples. This work was supported in part by CICYT (CGL 2007-63748/BOS) and CANTUR S.A. Felipe Martínez-Pastor was supported by the Ramon y Cajal program (Spanish Ministry of Education and Science).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Nicolas, M., Alvarez, M., Gomes-Alves, S. et al. Effects on brown bear (Ursus arctos) spermatozoa freezability of different extender and dilution ratios used for pre-freezing centrifugation. Eur J Wildl Res 57, 259–266 (2011). https://doi.org/10.1007/s10344-010-0420-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-010-0420-y