Abstract
Objectives
Contrast agent (CA) relaxivities are generally not well established in vivo, and the relationship between frequency/phase shift and magnetic susceptibility might be a useful alternative for CA quantification.
Materials and methods
Twenty volunteers (25–84 years old) were investigated using test–retest pre-bolus dynamic susceptibility-contrast (DSC) magnetic resonance imaging (MRI). The pre-bolus phase-based venous output function (VOF) time integral was used for arterial input function (AIF) rescaling. Resulting cerebral blood flow (CBF) data for grey matter (GM) were compared with pseudo-continuous arterial spin labelling (ASL). During the main bolus CA passage, the apparent spatial shift (pixel shift) of the superior sagittal sinus (seen in single-shot echo-planar imaging (EPI)) was converted to CA concentration and compared with conventional ΔR2*-based data and with a predicted phase-based VOF from the pre-bolus experiment.
Results
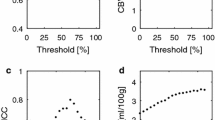
The phase-based pre-bolus VOF resulted in a reasonable inter-individual GM CBF variability (coefficient of variation 28 %). Comparison with ASL CBF values implied a tissue R2*-relaxivity of 32 mM−1 s−1. Pixel-shift data at low concentrations (data not available at peak concentrations) were in reasonable agreement with the predicted phase-based VOF.
Conclusion
Susceptibility-induced phase shifts and pixel shifts are potentially useful for large-vein CA quantification. Previous predictions of a higher R2*-relaxivity in tissue than in blood were supported.



Similar content being viewed by others
References
Rempp KA, Brix G, Wenz F, Becker CR, Gückel F, Lorenz WJ (1994) Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 193:637–641
Morell A, Lennmyr F, Jonsson O, Tovedal T, Pettersson J, Bergquist J, Zemgulis V, Einarsson GM, Thelin S, Ahlström H, Bjørnerud A (2015) Influence of blood/tissue differences in contrast agent relaxivity on tracer-based MR perfusion measurements. Magn Reson Mater Phy 28:135–147
Kiselev VG (2001) On the theoretical basis of perfusion measurements by dynamic susceptibility contrast MRI. Magn Reson Med 46:1113–1122
Kjølby BF, Østergaard L, Kiselev VG (2006) Theoretical model of intravascular paramagnetic tracers effect on tissue relaxation. Magn Reson Med 56:187–197
van Osch MJ, Vonken EJ, Viergever MA, Van der Grond J, Bakker CJ (2003) Measuring the arterial input function with gradient echo sequences. Magn Reson Med 49:1067–1076
Akbudak E, Kotys MS, Memisevic D, Conturo TE (2004) Quadraticity and hematocrit dependence of ΔR2* AIF signals at 3 T: a blood phantom study under physiological conditions. In: Syllabus of the ISMRM workshop on Quantitative Cerebral Perfusion Imaging Using MRI: A Technical Perspective, Venice, pp 10–11
Kalavagunta C, Michaeli S, Metzger GJ (2013) In vitro Gd-DTPA relaxometry studies in oxygenated venous human blood and aqueous solution at 3 and 7 T. Contrast Media Mol Imaging 9:169–176
Calamante F, Connelly A, van Osch MJ (2009) Nonlinear ΔR2* effects in perfusion quantification using bolus-tracking MRI. Magn Reson Med 61:486–492
Kjølby BF, Mikkelsen IK, Pedersen M, Østergaard L, Kiselev VG (2009) Analysis of partial volume effects on arterial input functions using gradient echo: a simulation study. Magn Reson Med 61:1300–1309
Bleeker EJ, van Buchem MA, van Osch MJ (2009) Optimal location for arterial input function measurements near the middle cerebral artery in first-pass perfusion MRI. J Cereb Blood Flow Metab 29:840–852
Knutsson L, Ståhlberg F, Wirestam R, van Osch MJ (2013) Effects of blood ΔR2* non-linearity on absolute perfusion quantification using DSC-MRI: comparison with Xe-133 SPECT. Magn Reson Imaging 31:651–655
Knutsson L, Börjesson S, Larsson EM, Risberg J, Gustafson L, Passant U, Ståhlberg F, Wirestam R (2007) Absolute quantification of cerebral blood flow in normal volunteers: correlation between Xe-133-SPECT and dynamic susceptibility contrast MRI. J Magn Reson Imaging 26:913–920
Zaharchuk G, Bammer R, Straka M, Newbould RD, Rosenberg J, Olivot JM, Mlynash M, Lansberg MG, Schwartz NE, Marks MM, Albers GW, Moseley ME (2009) Improving dynamic susceptibility contrast MRI measurement of quantitative cerebral blood flow using corrections for partial volume and nonlinear contrast relaxivity: a xenon computed tomographic comparative study. J Magn Reson Imaging 30:743–752
Blockley NP, Jiang L, Gardener AG, Ludman CN, Francis ST, Gowland PA (2008) Field strength dependence of R1 and R2* relaxivities of human whole blood to ProHance, Vasovist, and deoxyhemoglobin. Magn Reson Med 60:1313–1320
Patil V, Johnson G (2013) ΔR2* gadolinium-diethylenetriaminepentacetic acid relaxivity in venous blood. Magn Reson Med 69:1104–1108
Rausch M, Scheffler K, Rudin M, Radü EW (2000) Analysis of input functions from different arterial branches with gamma variate functions and cluster analysis for quantitative blood volume measurements. Magn Reson Imaging 18:1235–1243
Knutsson L, Lindgren E, Ahlgren A, van Osch MJ, Markenroth Bloch K, Surova Y, Ståhlberg F, van Westen D, Wirestam R (2014) Dynamic susceptibility contrast MRI with a prebolus contrast agent administration design for improved absolute quantification of perfusion. Magn Reson Med 72:996–1006
Calamante F, Vonken EJ, van Osch MJ (2007) Contrast agent concentration measurements affecting quantification of bolus-tracking perfusion MRI. Magn Reson Med 58:544–553
Newbould RD, Skare ST, Jochimsen TH, Alley MT, Moseley ME, Albers GW, Bammer R (2007) Perfusion mapping with multiecho multishot parallel imaging EPI. Magn Reson Med 58:70–81
Marques JP, Bowtell R (2005) Application of a Fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts Magn Reson B 25B:65–78
Wharton S, Schäfer A, Bowtell R (2010) Susceptibility mapping in the human brain using threshold-based k-space division. Magn Reson Med 63:1292–1304
Bonekamp D, Barker PB, Leigh R, van Zijl PC, Li X (2015) Susceptibility-based analysis of dynamic gadolinium bolus perfusion MRI. Magn Reson Med 73:544–554
Akbudak E, Conturo TE (1996) Arterial input functions from MR phase imaging. Magn Reson Med 36:809–815
Conturo TE, Akbudak E, Kotys MS, Chen ML, Chun SJ, Hsu RM, Sweeney CC, Markham J (2005) Arterial input functions for dynamic susceptibility contrast MRI: requirements and signal options. J Magn Reson Imaging 22:697–703
Foottit C, Cron GO, Hogan MJ, Nguyen TB, Cameron I (2010) Determination of the venous output function from MR signal phase: feasibility for quantitative DCE-MRI in human brain. Magn Reson Med 63:772–781
Garpebring A, Wirestam R, Yu J, Asklund T, Karlsson M (2011) Phase-based arterial input functions in humans applied to dynamic contrast-enhanced MRI: potential usefulness and limitations. Magn Reson Mater Phy 24:233–245
Bleeker EJW, van Buchem MA, Webb AG, van Osch MJP (2010) Phase-based arterial input function measurements for dynamic susceptibility contrast MRI. Magn Reson Med 64:358–368
Haacke EM, Brown RW, Thompson MR, Venkatesan R (1999) Magnetic resonance imaging: physical principles and sequence design. Wiley, New York
de Rochefort L, Brown R, Prince MR, Wang Y (2008) Quantitative MR susceptibility mapping using piece-wise constant regularized inversion of the magnetic field. Magn Reson Med 60:1003–1009
Lindgren E, Wirestam R, Markenroth Bloch K, Ahlgren A, van Osch MJ, van Westen D, Surova Y, Ståhlberg F, Knutsson L (2014) Absolute quantification of perfusion by dynamic susceptibility contrast MRI using Bookend and VASO steady-state CBV calibration: a comparison with pseudo-continuous ASL. Magn Reson Mater Phy 27:487–499
Young S, Bystrov D, Netsch T, Bergmans R, Van Muiswinkel A, Visser F, Sprigorum R, Gieseke J (2006) Automated planning of MRI neuro scans. In: Proc SPIE 6144:61441 M
Köstler H, Ritter C, Lipp M, Beer M, Hahn D, Sandstede J (2004) Prebolus quantitative MR heart perfusion imaging. Magn Reson Med 52:296–299
Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG (2003) Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 50:164–174
Srour JM, Shin W, Shah S, Sen A, Carroll TJ (2011) SCALE-PWI: a pulse sequence for absolute quantitative cerebral perfusion imaging. J Cereb Blood Flow Metab 31:1272–1282
Alsop DC, Detre JA (1996) Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 16:1236–1249
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Parkes LM, Rashid W, Chard DT, Tofts PS (2004) Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 51:736–743
Kilroy E, Apostolova L, Liu C, Yan L, Ringman J, Wang DJ (2014) Reliability of two-dimensional and three-dimensional pseudo-continuous arterial spin labeling perfusion MRI in elderly populations: comparison with 15O-water positron emission tomography. J Magn Reson Imaging 39:931–939
Bjørnerud A, Emblem KE (2010) A fully automated method for quantitative cerebral hemodynamic analysis using DSC-MRI. J Cereb Blood Flow Metab 30:1066–1078
Langham MC, Magland JF, Epstein CL, Floyd TF, Wehrli FW (2009) Accuracy and precision of MR blood oximetry based on the long paramagnetic cylinder approximation of large vessels. Magn Reson Med 62:333–340
Jain V, Langham MC, Wehrli FW (2010) MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab 30:1598–1607
Kellner E, Mader I, Mix M, Splitthoff DN, Reisert M, Foerster K, Nguyen-Thanh T, Gall P, Kiselev VG (2013) Arterial input function measurements for bolus tracking perfusion imaging in the brain. Magn Reson Med 69:771–780
Acknowledgments
This study was supported by the Swedish Research Council (Grants No. 2007-6079, 2010-4454 and 2011-2971), and the Swedish Cancer Society (Grant No. 2012/597).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Regional Ethical Review Board in Lund, Sweden. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10334_2016_567_MOESM1_ESM.tif
Graphical plots of the ΔR2*-vs-C relationships in Eqs. 3 and 4. Two previously published non-linear mathematical representations of experimental ΔR2*-versus-C data at 3 T were used for concentration quantification; Eq. 3 is based on in-vitro measurements in fully oxygenated whole blood [4, 9], and Eq. 4 is derived from in-vivo fitting using venous data [15]. (TIFF 28399 kb)
10334_2016_567_MOESM2_ESM.tif
CA concentration as a function of time in the superior sagittal sinus during a DSC-MRI bolus passage. Data correspond to one measurement in one representative subject. In order to make an unbiased choice of dataset, the measurement with the median pixel-shift-based tail concentration (cf. Fig. 1b) was used. Concentration was calculated from the apparent shift in vessel position (blue diamonds) as well as from ΔR2* values using the arterial blood representation (green squares, Eq. 3) and the venous blood representation (orange triangles, Eq. 4). Data points at baseline (zero concentration) and at peak concentrations (extinguished MRI signal) were excluded. The concentration estimates from the main bolus DSC-MRI experiment are compared with a phase-based VOF predicted from a separate pre-bolus experiment (dashed red curve). (TIFF 42620 kb)
Rights and permissions
About this article
Cite this article
Wirestam, R., Lind, E., Ahlgren, A. et al. Dynamic susceptibility contrast perfusion MRI using phase-based venous output functions: comparison with pseudo-continuous arterial spin labelling and assessment of contrast agent concentration in large veins. Magn Reson Mater Phy 29, 823–831 (2016). https://doi.org/10.1007/s10334-016-0567-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-016-0567-y